We will be focusing on paper chromatography for both parts of this
advertisement

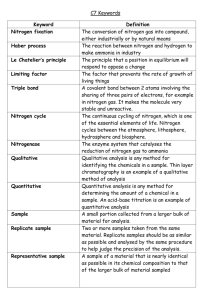
We will be focusing on paper chromatography for both parts of this lab. A small concentrated spot of the solution that contains the sample of the solute is applied to a strip of chromatography paper near the base of the plate. The sample is absorbed onto the paper, which is the stationary phase. The paper is dipped into a suitable solvent (mobile phase), such as water or alcohol, and is placed into a sealed container. The solvent moves up the paper by capillary action, which occurs as a result of the attraction of the solvent molecules to the paper. As the solvent rises through the paper it meets and dissolves the sample mixture, which will then travel up the paper with the solvent solute sample. Different compounds in the sample mixture travel at different rates due to differences in solubility in the solvent and due to differences in their attraction to the fibers in the paper. The more soluble the component, the further up it goes. Part A: Chromatography of Markers Procedure 1. Obtain three markers, one of each type (permanent, dry erase, magic). 2. Obtain three strips of chromatography paper. 3. In the center of each strip about 1 cm from one edge of the paper, draw a pencil line straight across the page. 4. Put a dot of the marker ink to be tested (2mm dot) 5. Using a graduated cylinder, pour enough water into a small beaker to cover the bottom of the beaker. 6. Carefully insert the chromatography paper into the beaker so that the water is below the pencil line. Do not let the dot touch the water line! 7. Allow the chromatography set-up to rest undisturbed for several minutes until the water is about 2 cm from the top of the paper. 8. Allow the chromatography paper to dry completely. 9. Repeat steps 3-8 for the remaining two markers. 10. Repeat steps 3-9 but replace the water with alcohol! At the end of this procedure, you should have 6 chromatography papers! If you don’t get the actual chromatograph to tape or staple into your lab book, you will have to draw a picture of it into your lab book. Every lab partner should have all 6 chromatographs drawn or stapled into the lab notebook. Calculation Table Marker Type 1 Solvent Water Alcohol 2 Water Alcohol 3 Water Alcohol Solvent Distance (cm) Color distance (cm) Rf Analysis 1. Measure the distance the solvent traveled from the starting point. This means measure from the pencil line to the water line. Record this value in the calculation table. 2. Measure the distance each pigment traveled from the starting point. This means measure from the pencil line to the top of each color streak. 3. Calculate the Retention Factor (Rf) for each pigment. To do this, divide the distance that the pigment moved by the distance that the solvent moved. Part B: Candy Color Chromatography Procedure 1. Cut a piece of chromatography paper into an 8cm x 8 cm square. 2. Draw a line with a pencil about 1 cm from one edge of the paper. 3. Make 6 dots with the PENCIL equally spaced along the line, leaving about ½ cm margins. 4. Below the line, use the PENCIL to label each color candy you will use. 5. Obtain a sample of candy color dye by soaking a candy in a SMALL quantity of water. 6. Take a pipette and pull a little bit of the colored liquid up. Put a really small dab of the colored liquid onto the corresponding labeled dot on your chromatopgraphy paper. 7. Allow the drops to dry. 8. Pour alcohol into a beaker to just cover the bottom. Do not let the alcohol line rest above the pencil line on your chromatography sheet. 9. Put the paper into the beaker and wait. 10. When the alcohol is about 1 cm from the top of the paper, remove the paper and allow it to dry. At the end of this procedure, you should have 1chromatography paper! If you don’t get the actual chromatograph to tape or staple into your lab book, you will have to draw a picture of it into your lab book. Every lab partner should have a chromatograph drawn or stapled into the lab notebook. Calculation Table Color Candy Solvent Distance (cm) Color distance (cm) Rf Analysis 1. Measure the distance the solvent traveled from the starting line. Record this value in the calculation table 2. Measure the distance each of the pigments traveled from the starting line. 3. Calculate the Rf values for each of the colors for each of the candies. Questions: 1. What color markers were mixtures from your experiments? Which markers were pure substances? 2. Which solvent worked best as a mobile phase for each marker? Why do you think there was a difference? 3. Why does the mixture spot need to be above the level of the solvent when the chromatograph is placed in the solvent? 4. From a comparison of the Rf values, what conclusion can be made about the similarities or differences in the dyes used to color each of the candies? 5. Obtain a candy package and determine if the food colorings used in the ingredients matches with your findings. Which color candies use mixtures of pigments to make their color shells? How can you tell? 6. If you were to do this experiment again, what would you do differently and what is one very detailed error that affected your lab?