IN.PACT Admiral Drug-Coated Balloon
advertisement
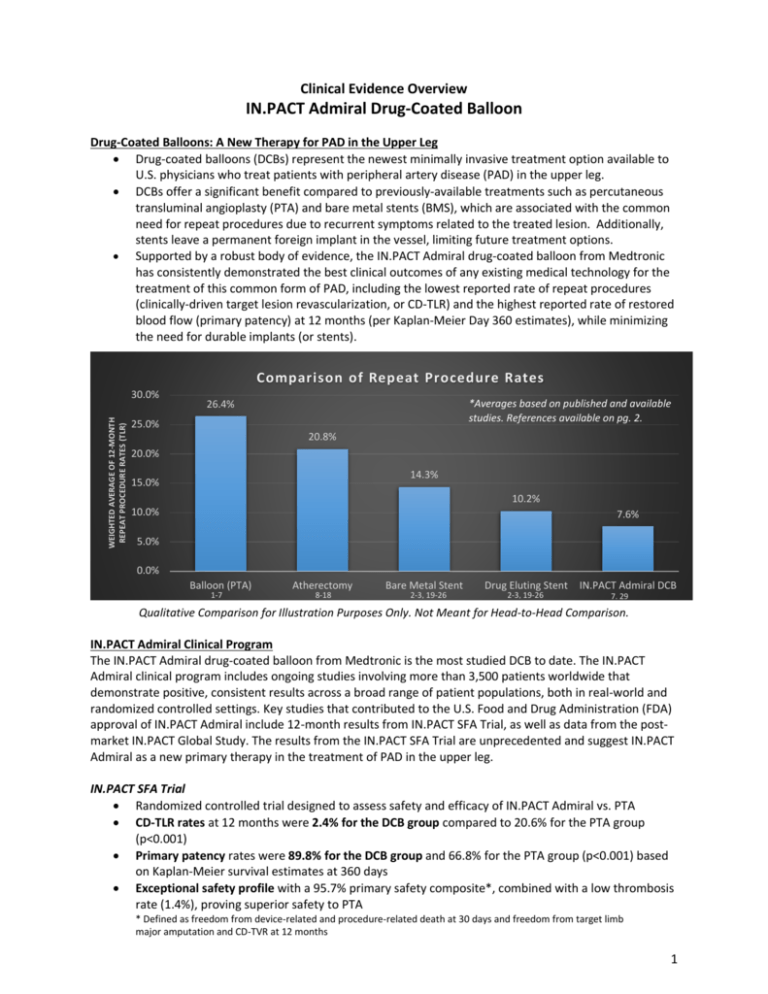
Clinical Evidence Overview IN.PACT Admiral Drug-Coated Balloon Drug-Coated Balloons: A New Therapy for PAD in the Upper Leg Drug-coated balloons (DCBs) represent the newest minimally invasive treatment option available to U.S. physicians who treat patients with peripheral artery disease (PAD) in the upper leg. DCBs offer a significant benefit compared to previously-available treatments such as percutaneous transluminal angioplasty (PTA) and bare metal stents (BMS), which are associated with the common need for repeat procedures due to recurrent symptoms related to the treated lesion. Additionally, stents leave a permanent foreign implant in the vessel, limiting future treatment options. Supported by a robust body of evidence, the IN.PACT Admiral drug-coated balloon from Medtronic has consistently demonstrated the best clinical outcomes of any existing medical technology for the treatment of this common form of PAD, including the lowest reported rate of repeat procedures (clinically-driven target lesion revascularization, or CD-TLR) and the highest reported rate of restored blood flow (primary patency) at 12 months (per Kaplan-Meier Day 360 estimates), while minimizing the need for durable implants (or stents). Comparison of Repeat Procedure Rates WEIGHTED AVERAGE OF 12-MONTH REPEAT PROCEDURE RATES (TLR) 30.0% *Averages based on published and available studies. References available on pg. 2. 26.4% 25.0% 20.8% 20.0% 14.3% 15.0% 10.2% 10.0% 7.6% 5.0% 0.0% Balloon (PTA) 1-7 Atherectomy 8-18 Bare Metal Stent 2-3, 19-26 Drug Eluting Stent 2-3, 19-26 IN.PACT Admiral DCB 7, 29 Qualitative Comparison for Illustration Purposes Only. Not Meant for Head-to-Head Comparison. IN.PACT Admiral Clinical Program The IN.PACT Admiral drug-coated balloon from Medtronic is the most studied DCB to date. The IN.PACT Admiral clinical program includes ongoing studies involving more than 3,500 patients worldwide that demonstrate positive, consistent results across a broad range of patient populations, both in real-world and randomized controlled settings. Key studies that contributed to the U.S. Food and Drug Administration (FDA) approval of IN.PACT Admiral include 12-month results from IN.PACT SFA Trial, as well as data from the postmarket IN.PACT Global Study. The results from the IN.PACT SFA Trial are unprecedented and suggest IN.PACT Admiral as a new primary therapy in the treatment of PAD in the upper leg. IN.PACT SFA Trial Randomized controlled trial designed to assess safety and efficacy of IN.PACT Admiral vs. PTA CD-TLR rates at 12 months were 2.4% for the DCB group compared to 20.6% for the PTA group (p<0.001) Primary patency rates were 89.8% for the DCB group and 66.8% for the PTA group (p<0.001) based on Kaplan-Meier survival estimates at 360 days Exceptional safety profile with a 95.7% primary safety composite*, combined with a low thrombosis rate (1.4%), proving superior safety to PTA * Defined as freedom from device-related and procedure-related death at 30 days and freedom from target limb major amputation and CD-TVR at 12 months 1 Deeper Look at IN.PACT SFA Trial Patient Population A review of select baseline characteristics is indicative of the high prevalence of co-morbidities and complications commonly observed in patients with PAD in the upper leg. These characteristics further increase the risk of heart disease, heart attack, stroke, and health complications related to diabetes that are associated with this challengingto-treat condition. Select Baseline Characteristics Age (years) Male Gender Diabetes Hypertension Coronary Artery Disease Carotid Artery Disease Current Smoker IN.PACT SFA Trial (n=220 IN.PACT Admiral subjects) 67.5 ± 9.5 65.0% (143/220) 40.5% (89/220) 91.4% (201/220) 57.0% (122/214) 34.9% (73/209) 38.6% (85/220) IN.PACT Global Study Largest post-market registry of its kind designed to assess safety and efficacy of the IN.PACT Admiral in real-world clinical practice Reproducible, positive outcomes were observed in this study, which included patients with more complex disease and more challenging lesions Results from the first 655 patients treated in IN.PACT Global include a 12-month CD-TLR rate of 8.7%, which validate the findings from IN.PACT SFA The data reinforce strong safety of IN.PACT Admiral, with an 89.6% primary safety composite*, and low thrombosis rate (3.8%) * Defined as freedom from device-related and procedure-related death at 30 days and freedom from target limb major amputation and CD-TVR at 12 months Chart References 1. Tepe G et al. N Engl J Med. 2008; 358:689-99 2. Laird JR et al. Circulation. 2010; 3:267-76 3. Krankenberg H et al. Circulation. 2007; 116:285-92 4. Dake MD et al. Circ Cardiovasc Interv. 2011; 4:495-504 5. Werk M et al. Circ Cardiovasc Interv. 2012; 5:831-40 6. Scheinert D et al. JACC Cardiovasc Interv. 2014; 7:10-9 7. Tepe, G Charing Cross Symposium. 2014; London, UK 8. Ramaiah, V Semin Vasc Surg. 2008; 21(1):41-49 9. Kiesz, RS Catheter Cardiovasc Interv. 2013; 82(3):E244-250 10. Sixt S et al. Ann Vasc Surg. 2011; 25(4):520-529 11. Zeller T et al. J Endovasc Ther. 2009; 16(6):653-662 12. Dave RM et al. J Endovasc Ther. 2009; 16(6):665-675 13. Zeller T et al. J Am Coll Cardiol. 2006; 48(8):1573-1578 14. Biskup NI et al. Ann Vasc Surg. 2008; 22(6):776-782 15. Dattilo R et al. J Invasive Cardiol. 2014; 26(8):355-360 16. Shammas NW et al. J Vasc Interv Radiol. 2011; 22(9):12231228 17. Singh T et al. J Invasive Cardiol. 2011; 23(7):269-273 18. Regine R et al. Radiol Med. 2010; 115(8):1208-1218 19. Bosiers M et al. J Endovasc Ther. 2009; 16:261-9 20. Diehl SJ et al. J Vasc Interv Radiol. 2012; 23:1317-22 21. Banerjee S et al. J Am Coll Cardiol. 2012; 60:1352-9 22. Rastan A et al. Circulation. 2013; 127:2535-41 23. Tadros RO et al. Annals of Vascular Surgery. 2014; 28:1-9 24. Werner M et al. J Endovasc Ther. 2013; 20:759-66 25. Duda SH et al. J Endovasc Ther. 2006; 13:701-10 26. Kralj I et al. VASA Zeitschrift fur Gefasskrankheiten. 2013; 42:340-9 27. Lammer J et al. J Vasc Surg. 2011; 54:394-401 28. Dake MD et al. J Endovasc Ther. 2011; 18:613-23 29. Ansel, G Transcatheter Cardiovascular Therapeutics. 2014; Washington, DC 2





![Best-fit Decisions [, 25 KB]](http://s3.studylib.net/store/data/006613263_1-62a61f810693250e9828b38f77c693b2-300x300.png)