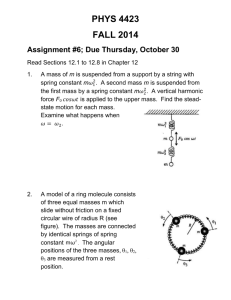
Experiment 1: Report form

Synthesis of Tris(2,4-pentanedionato)chromium(III)
Name: _______________________ Date: ________________________
Partner(s): ________________________________
Use complete sentences, use the proper number of significant figures, and include units. We completed the Introduction and Theory for you to act as examples for subsequent lab reports. We also completed parts of the Procedure and Results to help get you started.
Remove all the italicized prompts in your final report.
Introduction: Tris(2,4-pentanedionato)chromium(III) is synthesized and the purity is determined using mass spectrometry.
Theory: The m/z values for the expected masses of the most abundant peak in the isotope cluster for the substance and for impurities were calculated using literature values of the masses of the isotopes of the elements using Table 1 in the lab write-up.
1
Isotope averaged molar masses were calculated using the Periodic Table.
2
Procedure: Tris(2,4-pentanedionato)chromium(III) was synthesized by the reaction of CrCl
3
6H
2
O with 2,4pentanedione in basic, aqueous solution using a previously published procedure.
1
Add the remainder of the “Procedure” as request5ed in the lab write-up
:
Results: Present the results of your calculations : The mass spectrum showed a range of impurities along with the desired product, Figure 1. The product peak appeared at ____________m/z and __ number ___ impurities were found. The impurities are (give the list) .
For the most abundant peaks in each isotope cluster, the experimentally determined monoisotopic masses, theoretical masses, errors in ppm, and isotope averaged molar masses are given in Tables 1 and 2.
Table 1: Masses for Protonated tris(2,4-pentanedionato)chromium(III) and Impurities
Ion Experimental mass Theoretical Mass Error (ppm) Molar Mass (g/mol)
Add the remaining results requested in the Results section of the write-up :
Discussion: (1). Purpose accomplished: Essentially restate the purpose of the experiment, but as completed goal:
(2). Discuss the accuracy of the determination of the monoisotopic molar masses of the most abundant peak of the isotope cluster from your substance:
(3). Was the factor that dominated the accuracy of your MS results a random or systematic error? Describe how you decided:
(4). Describe why the monoisotopic molar mass of a substance and the periodic table based molar mass are different:
(5). Identify the major impurities in your sample:
(6). Give two possible sources of systematic error in your percent yield:
(7). Based on your MS, and percent yield, were you successful at making the Cr(acac)
3
complex?
Literature Cited
1. Honors General Chemistry, “Experiment 1: Synthesis of Tris(2,4-pentanedionato)chromium(III),” www.colby.edu/chemistry/CH145/lab/CH145(1)Cr14.pdf
, last accessed 8/28/2014.
2.
(add the source for periodic table used in the Theory section:)
Please attach a copy of your MS spectrum with a caption as Figure 1.
Please attach a copy of your spreadsheet with a caption as Table 2.
Captions start with Figure # or Table # and then a concise description of the contents.
You can write the captions by hand in black pen.
Remove all the italicized prompts in your final report.
Pay attention to the parts of this report that we wrote. You will be on your own in subsequent reports.