Density Stations Lab
advertisement

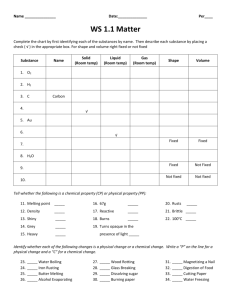
Density Stations Lab Teacher notes: Have students fold lined paper in half so they can use front and back to record observations and answers to questions for each station in a “block”. One class period for just 4 stations. Double if you do 8 stations. Quadruple if you do 8 stations & 2 periods. Expect station 1 to take longer than others. Station 1: Density of a cube Materials 3 solid prisms (cornstarch, baking soda, & Dixie cup boxes work great) 1 ruler 1 scale 1 calculator Station 2: Displacement Method of Finding Volume Materials 3 small rocks 1 100 ml graduated cylinder 1 400 ml beakers 1 calculator water Station 3: Density of an Odd Shape Materials Grease pencil Action figure Scale 100 ml graduated cylinder 1 600 ml beakers water Station 4: Solid vs. Liquid Density Materials 1 600 mL beakers (used cubes) 5 300 mL beakers 5 plastic Ziploc bags (for cubes; re freeze between rotations) 5 plastic cups (for cubes at start of each rotation) Paper towels will be enough for 2 periods & 8 stations: 5 ice cube trays (freeze night before) 1 liter soda ½ gallon milk ½ gallon Juice ½ gallon water 1/gallon coffee, cooled Density Stations Lab Station 1: Density of a cube Directions: You will need to answer the questions below on a piece of lined paper. You do not need a separate paper for each station. To earn full credit your work must be neat with proper header and format. 𝑚𝑎𝑠𝑠 Density = 𝑣𝑜𝑙𝑢𝑚𝑒 D= 𝑚 𝑣 1. Make this data table on your paper. Prism 2. 3. 4. 5. Volume = length x width x height m v lxwxh V=lxwxh D Mass: using the scale measure the mass of each prism; record results in data table Volume: measure length, width, and height of each prism; multiple results and record in data table Density: for each prism divide mass by volume; record results in data table 𝑚 Solve: D = 𝑣 material a: mass = 3.6 grams volume = 12 ml density = ______ 𝑔𝑟𝑎𝑚𝑠 𝑚𝑙 material b: mass = 8.1 grams volume = 9 ml density = ______ 𝑔𝑟𝑎𝑚𝑠 𝑚𝑙 material c: mass = 7.2 grams volume = 0.36 ml density = ______ 𝑔𝑟𝑎𝑚𝑠 𝑚𝑙 6. If a solid is less dense than a liquid, the solid will float. From step 5 which material will float in water (water has a density of about 1.0)? Density Stations Lab Station 2: Displacement Method of Finding Volume Directions: You will need to answer the questions below on a piece of lined paper. You do not need a separate paper for each station. To earn full credit your work must be neat with proper header and format. You cannot use a ruler to measure the volume (length x width x height) of an irregular shape, such as a rock. That is why we use the displacement method. 1. Make this data table on your paper. Rock 1st Volume 1 2 3 2. 3. 4. 5. 6. 2nd Volume Rock Volume Fill graduated cylinder about half full with water. Record volume of water in data table. Put one rock in the cylinder. Record the new volume in data table. Pour out rock and water in beaker. Remove the rock from water and set aside. Repeat steps 2 – 4 for the remaining two rocks. If you subtract the 1st volume from the 2nd volume for each rock you will find the volume of the rock. Complete the last column of your data table. Density Stations Lab Station 3: Density of an Odd Shape Directions: You will need to answer the questions below on a piece of lined paper. You do not need a separate paper for each station. To earn full credit your work must be neat with proper header and format. You cannot use a ruler to measure the volume (length x width x height) of an irregular shape, such as a rock. That is why we use the displacement method. 1. 2. 3. 4. 5. Using scale measure the mass of the action figure. Record your measurement. Fill one beaker about halfway with water. Using grease pencil, make a small mark at the water level (volume). Submerge action figure in water and mark the new water level (volume). Remove action figure and set aside. Fill graduated cylinder, to top mark, with water. Slowly pour graduated cylinder’s water into beaker until the water level is back to the top mark. 6. Answer questions on your paper a. How much water (volume) did the graduated cylinder 1st have? b. How much water is left in the graduated cylinder? c. How much water did you pour from the graduated cylinder into the beaker? d. What is the volume of the action figure? 7. Pour out water and rub marks off the beaker. Density Stations Lab Station 4: Solid vs. Liquid Density Directions: You will need to answer the questions below on a piece of lined paper. You do not need a separate paper for each station. To earn full credit your work must be neat with proper header and format. If a solid is less dense than a liquid, the solid will float in the liquid. You are going to hypothesize which substance, when frozen (solid), will float in their liquid state. Remember a hypothesis is an, if then statement made before your experiment (also known as an educated guess). 1. Make this data table on your paper. If I put the solid for of a given substance in its liquid state Substance Water Milk Soda Juice Coffee, cooled Olive Oil then it will… Sink Float Hypothesis correct? y/n 2. For each substance, make a hypothesis about whether the solid state will sink or float in the liquid state. 3. One at a time, carefully place one solid state substance in its liquid state. Record if it your hypothesis was correct or not. 4. Carefully remove the used ice cubes and place them in the beaker labeled “used”. Use paper towels to clean-up. 5. When a lake freezes, the frozen portion is on top. Does this experiment demonstrate why? Explain your answer. 6. We can ice skate on a lake of frozen what because the top portion is what freezes first. If a lake was made of any of our other substances and frozen could we skate on any of them? Explain your answer.