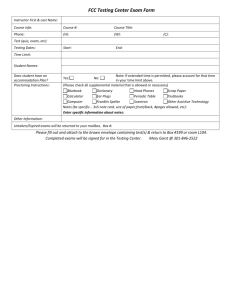
CHM-2046-SU2015-Rex
advertisement

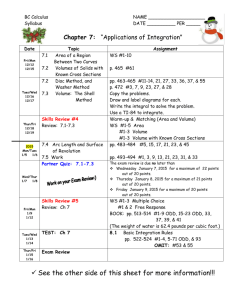
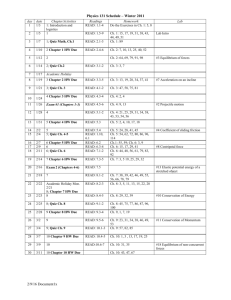
CHM 2046 Syllabus Summer 2015 11:00-11:50 a.m. M, T, W, R Instructor: Dr. Matthew Rex matthew.rex@ucf.edu Office: CH 208 Office Hours: M, W 1:00-2:30 pm T, R 9:30-10:30 am Textbook: Chemistry A Molecular Approach 3rd edition, Tro Course Goal: To provide a basic understanding of the chemical theory of kinetics, chemical equilibria, acid-base chemistry, electrochemistry and nuclear chemistry. Topics Covered: Topics include, but are not restricted to: intermolecular forces, properties of solutions, chemical kinetics, chemical equilibria, acids and bases, buffer solutions, precipitation reactions, thermodynamics (entropy and free energy), electron transfer reactions, and nuclear chemistry. Grading: Grades will be based on quizzes, four exams given throughout the class and a final exam on the last day of class. Grades will be assigned based on the following percentages: 90% - 100% = A, 80% - 89% = B, 70% - 79% = C, 60% - 69% = D, < 60% = F. Exams will be 50 minutes each (class time) and will be worth 100 points. Exams will be computergraded multiple choice format. You will NEED to bring a scantron for each exam. NO GRAPHING CALCULATORS ARE ALLOWED!!! NO USE OF ANY ELECTRONIC DEVICES (iPods, cell phones, MP3 players, computers, etc.) DURING EXAMS. Any use of such device will be considered cheating and result in a grade of 0 for that exam. A total of 7 discussion section quizzes will be given worth 20 points each. The two lowest quiz scores will be dropped. The final exam is on the last Wednesday & Thursday of class. It will be the normal class time of 50 minutes and will be cumulative. The final exam will replace your lowest exam score. Grading Summary 5-Quizzes 100 points 4-Exams (100 points each) 400 points 1-Final Exam 200 points Total 700 points A student ID will be required for each exam. Calculators will be needed for the exams and the final. All questions will be taken from material covered in the lecture, assigned reading, your textbook and textbook questions. Exams, including the final, will be computer graded. It is the responsibility of the student to have a clean, flat, pink SCANTRON test form and a number 2 pencil for each exam. Without the test from, students will not be allowed to take the exam. The instructor reserves the right to modify the schedule, testing procedure, and the grading basis if, in the professional judgment of the instructor, such modifications are in the best interest of fulfilling the course objectives and assuring the academic integrity of the course and institution. SCHEDULE Week Chapters May 18-21 Chapter 11: Liquids and Intermolecular Forces (Quiz 1 Thurs) May 25-28 Chapter 12: Solutions (Quiz 2 Wed) June 1-4 Chapter 13: Chemical Kinetics (Exam 1 Mon) June 8-11 Chapter 13: Chemical Kinetics (Quiz 3 Wed) June 15-18 Chapter 14: Chemical Equilibrium (Quiz 4 Wed) June 22-25 Chapter 14: Chemical Equilibrium (Exam 2 Tues) June 29-July 2 Chapter 16: Aqueous Ionic Equilibrium (Quiz 5 Wed) July 6-9 Chapter 16: Aqueous Ionic Equilibrium (Exam 3 Tues) July 13-16 Chapter 17: Free Energy & Thermodynamics (Quiz 6 Wed) July 20-23 Chapter 18: Electrochemistry (Quiz 7 Wed) July 27-30 Chapter 18: Electrochemistry (Exam 4 Tues) Aug 3-6 Chapter 19: Nuclear Chemistry Aug 5, 6 Final Exam VAB 132 11:00-11:50 a.m. Make up exams or quizzes are only allowed in the instances of illness, military service or jury duty and any of the 3 will require signed and dated documentation. Please provide me as much notice as possible in all cases. Make-ups will be written and taken in my office during office hours. Any make-ups should be taken in a timely fashion within a week of the absence. Tools for Being Successful Read the Chapters Before the Lecture Study Groups with Other Students Work End of Chapter Problems SI Sessions SARC Tutoring My Office Hours Private Tutoring – the Chemistry Office in PS #255 should have a list of private tutor names, emails and phone numbers. Holidays: Memorial Day, Monday May 25th Withdrawal Deadline: Monday, July 6th Independence Day, Friday July 3rd