Membrane and Transport
advertisement

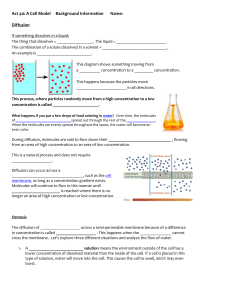
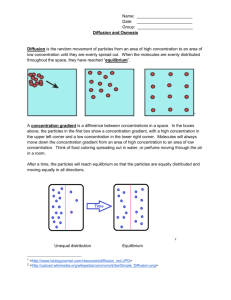
Notes for Diffusion, Osmosis and Transport across the Cell’s Plasma Membrane Recall That the Membrane Maintains Homeostasis The cell membrane is the most important part of the cell in regard to homeostasis of substances. The membrane is semi-permeable, also called selectively permeable, as it will not allow just anything into the cell. The regulation and maintenance of a steady balance of substances in the body is referred to as homeostasis. Cells must constantly maintain their pH in order to function properly. Your blood maintains a pH of about 7.4 which is slightly basic (alkaline), but maintaining this pH balance is crucial to life. Lysosomes, however, work properly in an acidic environment; a pH of about 5. _______________________________________________________________ Solute particles dissolved in solvent (like sugar, salt, etc) Solvent liquid in which solute dissolves; we’ll be looking at water as a solvent in this unit. Solution Mixture in which solute is evenly distributed (dissolved) in a solvent. An example is saline solution for cleaning contact lenses. NaCl (sodium chloride) is totally dissolved in water. Passive Transport of Solute and Solvent; Osmosis and Simple Diffusion During passive transport, particles move across the membrane; either into or out of the cell without using any energy (no ATP required). Diffusion and osmosis are both forms of passive transport. Simple Diffusion Particles move across membrane (either in or out of the cell) from an area of high concentration to an area of low concentration. Small particles and molecules such as O2, CO2 and H2O diffuse directly across the phospholipid bilayer. Concentration gradient Difference in solute concentrations on each side of the membrane. This illustrates particles moving with, or down, the concentration gradient; requires no energy The illustration above shows particles moving with the concentration gradient (high to low) 1 Water Flows via Osmosis in Response to Solute Concentration Osmosis The diffusion of water across the membrane from an area where there is more water (thus less solute) to an area where there is less water (thus more solute). Water flows with the concentration gradient in response to how much solute is dissolved in it; from a less concentrated solution, called hypotonic, into a more concentrated area, called hypertonic. Water will continue to move until equilibrium of concentration of solution on both sides of the cell is reached. Dynamic equilibrium is the term used as water will move in and out of the cell at a constant rate. Cells in a hypertonic solution higher concentration of solute outside of the cell (thus less water). Water will flow (osmosis) outside of the cell causing it to shrivel. Water flows from hypo- to hyper !! 2 Cells in a hypotonic solution lower concentration of solute outside of the cell (thus more water). Water will flow (osmosis) into the cell and it will swell (maybe burst if too much pressure). Water flows from hypo- to hyper !! Cells in isotonic solution (Normal state); Solute concentration is equal on both sides of the cell. Water will flow in and out at a constant rate (dynamic equilibrium). Remember molecules are constantly moving! 3 Osmotic Pressure The osmotic pressure of a solution is the pressure needed to prevent osmosis from occurring. Although U-tube B looks uneven, the concentration of solution is the same. Think of it this way. You can have a gallon of solution that contains 5% salt and a cup of solution that contains 5%.......Same concentrations, just different volumes of solution!!! See the illustration above. Water flows from low osmotic pressure (dilute solution) to high osmotic pressure (concentrated solution). As solute concentrations on both sides equalize, the pressure will stop osmosis from continuing, 4 Transport across the Cell Membrane Continued Another form of passive transport is called facilitated diffusion. In this case, particles cannot simply diffuse across the membrane. They need the help of proteins in the membrane but NO energy is required! Molecules still move from an area of high concentration to an area of low. Down (or with) the concentration gradient! Allows diffusion of large, membrane insoluble compounds such as glucose and amino acids Highly Selective. For example, the glucose transport protein in the liver will carry glucose into the cell but will not transport fructose Particles such as ions may pass through channel proteins Substance binds to carrier protein Particles move from areas of high concentration to areas of low concentration. Simple diffusion compared to facilitated diffusion. Channel proteins as well as carrier proteins may be involved! Active Transport Requires Energy May involve carrier proteins (see below) Movement across membrane against concentration or electrochemical gradient o Particles move from area of low concentration to an area of high concentration! 5 Endocytosis Active Transport Cells engulf molecules or fluids Particle comes in contact with membrane, folds inward forming a Characteristic of white blood cells Exocytosis Active transport The transport of material out of a cell by means of a sac or vesicle Examples: transport of proteins like enzymes, hormones and antibodies out of cell 6