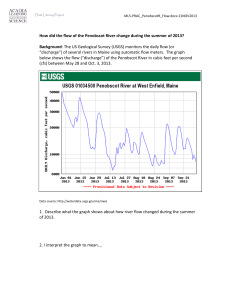
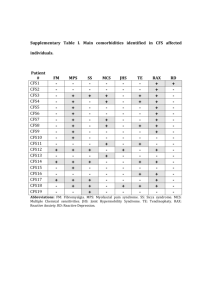
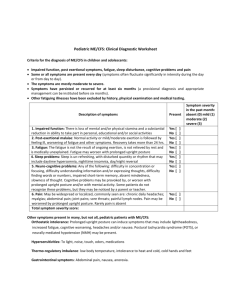
FDA Stakeholder Meeting on ME CFS, April 25
advertisement

FDA Stakeholder Meeting on ME CFS, April 25-26, 2013 Day 1 Test test. Please stand by. Please stand by. >> We're going to get started in about two minutes, if people can take their seats. Good afternoon, everyone, I'm delighted to be the person welcoming you to this part 1 of a two-day workshop. My name is Sandra Kweder, Deputy Director in the Office of Planning and Informatics at FDA and again welcome. Welcome to this important meeting on chronic fatigue syndrome and chronic myalgia. Let me make sure, nope, it changed my slide. We are really excited to be here ourselvess and we're excited that you're here. As I've looked over the attendance sheet, it's encouraging patient caregivers, patient advocates, drug developers, researchers and other people from the government all in the audience today. The think that binds us here today is our collective commitment to facilitate development of safe and effective therapies for what we all know is a very debilitating disease. The purpose of this meeting over the next day and a half is to explore important aspects that contribute to our thinking about the drug development process for CFS and ME. Our goal is to foster a shared understanding on the important issues regarding safe development of safe and effective therapies. For example, ultimately in identifying things like good measure of outcomes and clinical trials that would test new therapy, how to think about innovative ways to approach development in this disease. So let me see if I can manage the slides. Want to focus on what to expect. What we're hoping will come out of today and tomorrow, as well, but particularly today, a better understanding of what are the high impact signs and symptoms of this condition from the perspective of patients and those who care for patients. We'd like to gain some collective insight into clinical decisionmaking by healthcare providers who take care of these patients and have done so for years. What are their observations and what they see when patients improve or get worse and how is that match up with the perspective of patients? Where are those points of intersection that really seem to be places that we should focus on in thinking about developing new products? Similarly, some of this we've learned from clinical trials of drugs, large, small trials, practitioners experience and how do we apply those experiences going forward? Tomorrow we'll also hear some things about tools, whether they're scientific tools or regulatory tools to apply to drug development in this -- in these conditions and how we can merge those with the signs and symptoms that seem to matter most to patients. The way I see this, we are building a map, there are points on the map where there are pockets of great expertise, really heart felt positions or views on what matters most, really well studied, well thought out perspectives on how to think about this condition and monitoring it over time and how that would work into clinical development and then there's the scientific basis and understanding of patho physiology, clinical response, clinical trial outcome. How can we create the map that links all of those together? So in the way of objectives, we're here today on Day 1. And so let's start with the focus of that discussion. We're lucky that we have a whole collection of exerts here in the room, patients to launch our patient- focused drug development initiative. This initiative goes beyond the particular clinical conditions of CFS and ME, but we are thrilled that we can engage this community in launching this larger initiative at FDA. It's dedicated to engaging patients and patient representatives in dialogue, again on the most significant symptoms and negative impact experienced in their daily lives as a result of the condition. We seek their perspective on a range of therapy that they've been using or have used in the past to manage that condition and Dr. Theresa Mullin is going to explain more about that larger initiative shortly, right after me. Tomorrow's discussion will focus on things of a bit more of a technical nature and will explore the broader issues related to drug development. On Day 2, we'll cover topics such as clinical trial design, outcome measures, regulatory issues and possible pathways to expedite drug development for CFS and ME. But the focus of both days will be on common issues in drug development. Now it's natural that specific drug products are going to be raised, but they'll be raised in the context of the broader conversation about how to go forward. We're not here to discuss individual products that would be taken up to go further specifically although there may be references to that. You know, those more specifics and detailed discussions of going forward with individual drug products, we have different forms for those discussions that typically our advisory committee process are used for that, we can bring lots of data. Our goal is to move forward. So to just put us all in the same place, chronic -- we're talking about chronic fatigue syndrome or CFS, a serious complex and we all know severely debilitating disease, for the most part of unknown etiology, characterized by profound fatigue lasting six months or more by almost any definition one encounters and that is worsened by physical or intensive mental activity. We know it's a multi-system disorder with a variable symptom complex from patient to patient. There are no uniform diagnostic tests, which makes it really a challenging diagnosis and what clinicians often say tis diagnosis of exclusion and we know that there are no approved therapies. Complicating the picture further, there is a lack of consensus even among experts in the field on both nomenclature, and any specific or exact definition of the disease. I want to say a little bit about nomenclature, we'll talk about two terms. The term chronic fatigue syndrome and myalgic encephalomyelitis and the term drug development. First, regarding the name of the disease. We recognize that there's a lot of controversy in this area, some people call this CFS, some people call it myalgic encephalomyelitis, some people say that the conditions and the terms are referring to the same thing, some is people say that they're distinctly different. Okay. For purposes of this workshop, the terms are likely to be used interchangeably and used really as a frame of reference, okay. We and the people using them, we hope will make no judgment on the cause of the different symptom complex, their frame of reference. FDA doesn't endorse any particular definition, however, what we expect is that parties who are engaged in drug development who are submitting clinical trials for our review or new drugs where clinical trials have been conducted will articulate details about what definition they used for entering patients in the clinical trial so we can all understand who it is that we're studying. Okay. That's an important aspect that will probably come up more tomorrow and sort of the nuts and bolts of studying, testing drugs. Now again, let's go to drug development, for the purposes of this workshop, particularly today, we're using drug development in its broadest sense, the idea of identifying developing and evaluating potential therapies that can help patients manage their conditions and get better. We at FDA have a role in drug development with focus on ensuring safety in clinical trials and rendering decisions about whether or not a product safety and efficacy has adequately demonstrated for approval. If this workshop we're also going to be focusing on on the role of other key stakeholders in drug development, including pharmaceutical industry, academic researchers, clinicians and our other colleagues in the Department of Health and Human Services and in the patient community. So what's our agenda? Today we're -- the next speaker after me is Dr. Theresa Mullin, who will provide an overview of FDA's initialist on on patient focused drug development and following that Sara Eggers will go over the format and questions for the key topics being discussed today. During this discussion today when we hear from patients and their caregivers, some -- a few of my FDA colleagues and myself will be sitting up here and we may ask you some follow-up questions. They will be the follow-up questions like, can you tell me more what you meant about what you said in this part of your statement. And I'd like to ask my colleagues who are going to be part of that to introduce themselves. Why don't we start right here. >> Terry colego. >> Where do you work for FDA? >> CDER. >> Terry for those who don't know, has a really long history at the agency, she's a pharmacist and for a very long time Terry was in charge of all of our patient and patient advocate outreach input and output activities. She is a long track record of excellence in listening and making sure that people get heard. So theresa, number two, next to her. >> All right, I'm Thersa, Michelle in the division of pulmonary rheumatology product in the Office of new drug at FDA. Our division now is the home at FDA for all of the chronic fatigue syndrome applications and I am also the agency representative to the fax. >> Question: Thanks, Terry. Theresa number three. >> Theresa Mullin, Office of planning and inform attics in the center for drugs and I'm CDER's lead on the patient focused drug development initiative, you'll hear more about that in a minute and I have to say I've never been on a panel with three theresas before. >> They all feel the same way, except Terry is spelled wrong on her card. And finally. >> Lauri burkeOffice of new drugs and we look at measures of treatment benefit and how to describe the results of clinical studies to demonstrate benefit in the labeling. >> Really important part THF discussion. So the end, we have the afternoon is organized you see on the slide. You can see that there -- this will be broken up into two topics. Sara Eggers will explain that more in a few minutes. Saran is right here. What I'd like to do, though, holdup on on introducing the panel, I know you will do that. At the end of the afternoon, there will be an open public comment period for those who preregistered and been confirmed to speak. There is a sign-in sheet at the registration table, be sure to check in if you signed up make sure your name is on the table. They'll confirm that with you W. That, I'm going to turn over to Theresa Mullin to talk a little bit more about this first day patient input. Again, thanks for being here, thank you for your participation. >> Theresa Mullin: Okay. Okay. Well, yes and hi again. I'm going to talk, give you a brief overview of this patient focused drug development initiative. This is particularly special meeting for us in terms of this initiative that we're starting under the prescription drug user fee act of -that was reAUTH rised in 2012, this is the first such meeting that we'll be having and I can't imagine a more important place to start in trying to see how well we're able to get information. We think that this initiative is going to very -- really help FDA because we don't get as much direct engage SXMENT input in a general way from patients as we think would really benefit us. We thought this initiative would be important adjunct to our benefit risk framework effort that we have under way and our benefit risk assessment for new drugs involve five key considerations that severity of the condition, the degree of unmet need, how much do current therapies treat how well do they treat the condition, what clinical benefit is evidenced in the data that has been collected in trials, what are the risks that are identified in the safety data collected in trials and the question of whether there is risk management plan that can make sure the benefits outweigh the risks. Well, the first two factors, the clinical comprise what we're calling the clinical context. Severity of the condition and the degree to which it's not met and taken care of by current therapies. Patients have a unique perspective on this, of course, they are the ones who experience disease, they are the ones living with it, they are the ones who are taking the medicines and seeing how well they work or don't. And we realize that we'd really benefit from a more systematic approach to gathering that information from patients and not just doing it in the context of a particular drug application where great deal of conflict of interest screening of people. We can't get a comprehensive picture because of the issues around a particular application, so we thought it would be much better if we try to go at this by disease and engage all the key stake holders really to us primarily. We want to hear from patients and their caregivers as caregiving is a very critical or large part of that experience for the patient and the patient family to really get their perspective to help us understand the clinical context in that most direct from that most direct perspective. We sense that we'll get immediate benefit from hearing about this, capturing it, putting it in a report that will give to review divisions to reference and use when getting application or other issues for specific drugs in that disease area.It may also stimulate the development of other outcome measures that capture more meaningful patient meaningful aspects of the disease that could later be used in clinical trials to look at aspects of drug performance and see if they're meeting the needs patients have told us about. So this initiative under the PRINGZ drug user fee reAUTH risation involve commitment to do at least 20, at least 20 meetings in 20 different disease areas over five years and you know, despite whatever budget constraints we are undergoing right now, we're very committeded to doing the initiative in any case and at least 20 and so we really want -- these meetings are primarily for patients, we want others to listen. It's an opportunity for patients to talk and frankly for everybody else to listen and so that is the way we've set it up. We hope that we get patient advocates to participate, drug companies that are interested in developing drugs in this disease area, FDA reviewers and care givers and other stake holders, we hope will be there to listen. We began this process of patient-focused drug development last summer, we started talking to the review division to figure out how to get it underway. We realized early on that we wanted additional patient input. We've got other ongoing patient what we call patient consultation meetings we've been conducting to get input and perspective on the things we're thinking of doing as part of this initiative. Last September we published a Federal register notice with a set of candidate disease areas that our review division identified as set they thought these could be good candidates, almost 40 drugs on the list that would be good to elicit public comment to see what the public thought about the drugs we had identified. We had a public meeting in October to discuss those candidate of these areas and we opened a docket, we receive body 4500 comments to the docket and we've analyzed those comments, taken them back and talked to the review division where the drugs that were identified in the docket would be reviewed to go back and try to identify what would be the best set to start with, given we're only working with about 20 in these first effort, we see this as longterm project, we don't think we'll be doing it for only five years, we think this is something we'll want to continue to do well beyond this five-year time frame of Fidufa, this is a good start for us. We identify the state of disease for the first three years, 2013 to 2015 and those are posted on our website and we can tell you more about those later if you'd like and this is as I said our first area. So it is very exciting for us. How did we come up with the initial list and what kind of diseases did we think would be particularly good candidates? You see, a list of areas, these are criteria we had used to develop the set and you can see why chronic fatigue syndrome is such a good candidate and such a good appropriate place to focus, for example our first disease area. We were looking in general for diseases that are chronic symptomatic and affect functioning and activity of daily living. One for which critical aspects for patients are not maybe captured as well as they could be in clinical trials, where there may be no currently effective therapy and no therapy approved for that disease, where there is range of severity that patients experience, there may be identical viable sub-population that have a particular experience with the disease that would help to hear more about and where there is fairly broad range of patients that may be affected by it. And as a set of 20, we wanted to try to cover wide range of disease areas to make sure that we were broadening our perspective and trying to get as many different diseases in there as we could. And so with that, we picked a set we think still corresponds to that for the first three years and in planning these specific meetings we've had sessions with as I mention patient consultation sessions we've had to go over the wording of the questions we came up with and patients that we had given us input on our initial questions greatly improved our questions and made them much more meaningful and in terms of how they were worded and what we were trying to get at. They were extremely helpful and helping us improve the wording and yet the same groups told us, you know, you'll continue to modify these questions, you'll have to for each group that you meet with because every disease is different and might need slightly different questions, may have different issues and you know, you need to be sensitive to that and we think that was very good advice and we have tried to do that, as well. Not only are we needing to modify the questions a little bit to fit the disease context that we'll be doing in a particular meeting, but we also want to be sure that the format of the meeting is tailored to sort of maximize the opportunity to get input from patients and to hear what they have to tell us in the course of a meeting. So we might vary our format for the meeting across the 20 a little bit for those reasons. We're hoping patients will participate and come to these meetings that we have if they can. We're also making sure that we have remote access available so people can also send in comments to electronically. We've heard from patients they would want to be sure we hear directly prosecute them and not be filtered through advocacy groups, so we're trying to mick sure we're providing ways for that to happen. We're looking at whether we can possibly expand and beyond the 20 maybe we can be collecting information electronically and doing other things for groups that we're not able to meet with face-to-face. Any of these meetings that we have and the information we will collect will be building into a report, which reports back what we've heard will be posting those reports on our website and we see that as the first product of perhaps a series of continuing efforts in each of the disease areas that we'll be looking at. And so if you'd like to hear more about it, there is a link to the website where you know almost like the first place to go look, we have a lot of information at this location and you can find it by going to this link. And with that, I really look forward to listening to your input today and thank you again for coming to the meeting. I'll turn it over to Sara Eggers now. >> Sara Eggers: Good afternoon. I'm the only one without a name card. Since I put the tent cards out, I don't know how I could have forgotten myself, but I did. It's really great to see you all here. We have been preparing for this for a long time and I'm so excited that the day has finally come and we get to hear the patients input directly. As Theresa, said, I'm Theresa Mullin, one of Dr. Mullins staff from the Office of Planning and Informatics within the Center for Drug Evaluation and Research. I will serve as discussion facilitator today and my colleague Theresa Toigo will support our discussion as moderator making sure the process runs smoothly, on on time, etcetera. This discussion is rather different from the types of government sponsored public meetings you participated in, so I'll be going over that format in the questions before we begin. We have two main topics today. The first topic is to explore the most significant symptoms of CFS and ME/CFS and impact on daily life. Here we want to know the most significant symptoms that you experience resulting from your condition, knowing that there are wide range of symptoms experienced. We also want to explore the negative impact in your daily life that result from those symptoms, specifically the important activities that you cannot do at all or fully as you would like because of your symptoms and because of your condition. Then the second topic is understanding patient perspective on treating CFS and ME. What are you currently doing? What is range of therapies you are currently using to treat your condition or its symptoms and how well do the treatments address your most significant symptoms? And what are the down sides of those treatments? Okay. We'll -- we have questions that will be coming up at the start of each discussion topic and that will give you a good sense. Now for the format. For each of the topics one and two, we're first going to hear from a panel of patients and patient representatives and the purpose here is to set a good foundation for our discussion. There are five panel discussions for each topic, primarily patients, but we do have a patient representative or caretaker on at least one on each of the panels. Each panelist has prepared two to three minutes of remarks in response to the questions that I'll put up. After we hear from each of them, weville time for follow-up questions for me and my FDA colleagues up here. After the panel discussion, we'll dialogue to include patient and patient representatives in the audience and the purpose here really is to build on the experiences shared by the panel and get a sense for what is generally similar and what may be different from what you heard. We'll focus on key questions for each topic and I'll be asking follow-up questions inviting participants to raise their hands to speak and I'll call on you and we have some people with microphones who will come to you. Okay. In a group this size, we will need to rely on this method of raising your hand to speak. I will try to get everyone who wants to speak knowing that we do have the time limitations, but as much as possible every one will have a chance to speak. Participating by live web cast can add comment through the webcast comment box and although they won't be read or summarized, they will be considered part of the public record.This meeting is being transcribed and we have people on our team taking detailed notes, as well. So even if you don't see me taking notes, I'm pretty bad note taker, I can't read my writing, just know others in the room are listening very intently and taking notes. Any point if you have to get up for any reason, please feel free, there are comfortable chairs near registration, there is piano there, as well, if anyone wants to play perform during the break, be my guest. There are restrooms located behind me, if you go out to the right here and keep going. We'll take a FEN-minute break at about 2:40. Around 2:40, between topics 1 and 2. In order for this meeting to add the most value to FDA and to the participants, we have a few ground rules for all of us. We are here first and foremost to hear directly from those who have this condition. Some of you are here today as caregivers representing someone who cannot be here in person and this is very important pause it means that we get to hear perspective from someone who may be too ill to travel today. We encourage you to contribute to the dialogue, as well. Some of you are here as patient advocates, we encourage your participation as well with the intent of speaking on behalf of patients. We are happy to see patients who represent government agencys and hope this input is important for you, as well, and you will learn something today. We ask that you stay in listening mode and let the patients and patient representatives contribute to the discussion. The purpose of the opening panel is to give our discussion solid foundation, however views expressed by participants speaking first will have no greater weight than any other comment expressed by participants. In this large group facilitated discussion, after the panel discussion we open up to large group, we'll ask participants to focus on the topics we're currently discussing, for example, a particular symptom. Try to keep responses or comments to a question to one or two minutes to ensure everyone can talk and focus on building on what others are saying. I will regularly ask for show of hands if you generally share a particular view or not and if you are comfortable, please raise your hand. We'll try to accommodate everyone who wants to speak. If we don't get your full thoughts on the topic we strongly encourage you to elaborate on your comment and what you heard in the docket which will remain open until August 2. Our discussion will focus on the common ground, regarding symptoms, impact and treatment of CFS and ME as Dr. Sandra Kweder said. There are many important issues to ensuring people with E-V-V-O CFS and ME get treatment they need. For our discussion we want to focus on topics I just went over, that is what is critical input for FDA at this time. We may touch upon specific treatments and that's okay, the discussion of any specific treatments however should be done in a way to help us understand the broader issues. Again, you are encouraged to elaborate on your perspective through the docket. And the comments on other topics are welcome at the open public comment for those who preregisterd and confirmed to speak during the session. FDA staff is here to listen. FDA will have its turn tomorrow to present and discuss in detail various issues related to the drug development process and they may build on as will all participants we hope on the various topics raised today. Occasionally as I said I will turn to them and ask if they have any questions or clarifications. We want your feedback from this meeting. What we learn will help us continue to design and implement future patient focused drug development meetings that are use to feel FDA and useful to those who contribute to these meetings. Evaluation forms are available completely voluntary and at the break if you go to the back table, I'll tell you at the break where to go. You can pick those up and we'd be happy to hear your comments. Above all, courtesy and respect is paramount, business our goal is to enable fair discussion, please wait to be acknowledged before speaking, speak into the microphone and please state your name. I'll try to call on people by name if I can read your name tags, but if you are able, and can stand, please stand and state your name. If you are not comfortable or not able to stand, please don't worry, you definitely don't need to. Avoid any kind of megative or degoratory language and keep side conversations to minimum so we can focus on the person who is contributed at the moment. Okay. With that, I think we're ready to begin discussion topic 1. I've asked the panelists for discussion topic 1 to join me at the front and they are here. I'll go through and say their names. And then I'll ask each of you and go through alphabetical order and ask you to share your remarks and then we'll have you follow- up questions. We have Dr. John Kiser, Mr. Joseph lansing, Denise LOpez, mayano, Mrs. KIM Mccleary. And Mrs. Charlotte BONsales. We have John kiser, Joseph Lansing, Denise lopezmayano, KIM Mccleary, and Charlotte BONsales. Thank you, with that, John, I will let you start. That is fine. >> Good afternoon, going to be a challenge to condense my experience with this condition into just a few minutes, but I'll do my best. My name is Dr. John Kiser, I practice medicine in the San Francisco Bay area N. 1987, after many stressful years of pre-med medical school residency training and working as a solar doc in a busy emergency room, I developed ME CFS. I had severe fatigue, recurring sore throats, chronic pain, and unrefreshing sleep. If I exercise just a modest amount, I would experience a devastating crash with exacerbation of all symptoms. I never knew how long a crash would last, sometimes a few days and other times several weeks. My ME CFS had significant effect on my work and my personal life. I had to cut down my work hours, I remember lying down on the couch frequently between patients and if I was able to socialize, I never knew how long it would be before I went up to my wife and said, we need to leave now. Like you just hit a wall and you become dizzy and light headed and your brain clouds over. Fortunately after five or six years of working really hard to rebuild my health, including taking a job with much less stress, and leaving emergency medicine, I was able to recover to a significant degree. However, I still experience relapses of these symptoms that significantly impact my life. When relapse occurs, I cannot participate fully as a father or a spouse. I need to lie in bed for hours at a time and I experience total body pain during these relapses. Now there are a lot of people who were totally disabled from this condition, bed bound or can't leave their homes. And there are lot worse off than I am, but I'm not here to speak for them, I'm here to speak for the thousands of people with CFS who are able to work, but struggle to make it through each day and each week. People who are able to work with CFS struggle to get our minds clear enough to function and to be able to think well enough to perform. We struggle to make it into work by 9 or 10:00 in the morning and be as productive as everyone else and we struggle to make it through an eight-hour workday without having to lie down and take a break. The worse part about this condition from my point of view is person with CFS who is still able to work is the toll this disease takes on my family. I don't know if I can convey the psychological distress I feel when I'm unable to spend time with my daughters or participate in family activities because I'm in bed on the weekend recovering from all the energy I expended during the week. This for me is the worst part of this condition, the fact that during exacerbation, I have no energy left to participate fully in my family and social life and one other terrible thing is which I'm sure all of you are familiar with is that family members and friends just can't understand how I can feel so sick when I'm at home if I'm still able to work and hold a job. I want to thank the FDA for holding this meeting and for getting input from patients themselves. I hope they will work to identify treatments that not only help those that are bed bound get out of the house, but also help those of us still able to work be able to think more clearly and function at a higher level. I look forward to hearing other people's perspectives and having a productive sharing of ideas. Thank you. (applause) >> Thank you very much, John. And I'm going to put the discussion questions up here so that you can see the types of questions they were addressing. We'll next move to Joseph Lansing. Thank you, Joseph. >> Hello, everyone, I'm Joe Lansen, I'm a veteran, I am a graduate student, sort of, and I have been ill for about eight years. A little bit about my life today. I live at home with my mom here in the DC metro area and I am on my seventh year of a two-year master's program. Lots of people take extra time to finish a graduate degree, but I'll tell you a little bit about my past so we can put my present situation in perspective. But first let me answer the central question, the symptom I chose is the most significant for me in my daily life is confusion. Which seems odd choice given the many different symptoms profoundly disabling for lots of us with the illness A Yes confusion? Several reasons. One, I now have trouble today for longer than 15 or 20 minutes to follow a single conversation with someone I know. My former life as veteran, military linguist, I translated arabic on the fly, typically listen to multiple conversations at once, one in each year of my head phone. I was on a plane, rattling plane, reconnaissance plain that wasn't very well pressurized, I was trapped in that for up to 10 hours. We would typically get up at 0200, 0400, 0 dark 30y as we know from the movie title and translate whatever our languages were on that plane for a long time. Then we would get up again and do it all the next day. I now have trouble sitting in a conversation office translating the same language for more than probably two or at most four hours would be the maximum I could do. And that's why confusion has affected me the most. The loss of my ability as linguist and hence any possibility of employment in that area. The inability to finish my graduate school program. It's possible I could work again on low concentration job, but I have not tried in years, I'm just hoping to finish my thesis. The confusion probably wouldn't matter as much without another symptom of the illness called most exERTional malaise. It is not that we are confused, any effort, mental, physical or emotional makes us so exhausted any progress we've made for instance onsaid thesis, I read it the next day, what I've written and I have to decide what do I have to decide? It's not making sense to me what I wrote, now does that mean I was confused when I wrote it and the writing is actually no good and I can't make sense of it now or am I confused now and unable to read a perfectly good sentence? In a sense it doesn't matter because that means the previous day's work is probably wasted. Thank you. >> (Applause) >> Sara Eggers: Thank you, Joseph. Denise. >> Good afternoon. Thank you everyone for making the effort to be here. Thank you especially to patients who have used scarce energy and financial resources to be here. I have two children, my son Matthew got sick two days after his 12th birthday, that was over eight years ago now. He's now 20. My son alexander got sick seven years ago, he's nearly 22. Before they got sick they were actively engaged in academics, championship level swimming, fencing, theater, family, friends, travel, world affairs, those are things of the past. They've been house bound since they got sick. They've tried numerous medications but had virtually no improvement. Since they got sick I provided 24/7 care for them. They require care, I am house bound as they are. I leave home to get materials from the library, go to the grocery store, I attend neighborhood book GRUP once every 10 weeks or so. When I leave home usually to take care of my sons in terms of medically related appointments driving them to and fro. To be here today I made arrangements for full-time caregiver to come in and stay at my home with my sons with coverage from friends and neighbors, as well. However, those plans went up in smoke, the caregiver two weeks ago today had a heart attack, said care giver cannot travel at present, said care giver cannot undergo situations of stress, let alone take care of people 24/7, other than herself. So I had to punt, I tried my best, couldn't come up with other arrangements so my sons are upstairs in a hotel room with friends and care givers looking in on them. Their symptoms run the gamut of malaise, immune problem, intolerance, neurocognitive problem, manifestation myalgia, unrefreshing sleep and more. Both of my sons more than meet the Canadian criteria and international criteria for ME. The symptoms that are most frustrating to them are impaired executive function, impaired reaction time, keep in mind in swimming and fencing they were used to quick starts, agile movement, slow processing speed, impaired working memory, difficulty processing information. It was said earlier intensive cognitive exertion triggered post-exertional malaise FRSHGS my experience with my son it can be triggered by minimal exertion. My sons post exertional malaise can be triggered by cognitive exertion that lasts more than 20 minutes at a time about three times a week. Meaning they can't study for any expended period of time. The term malaise to the layperson is misnomer, what they experience and what most patients I know of experience is more like a collapse, as I said before. For my son, post-exertional malaise or collapse can be brought on by physical or cognitive exertion and these collapses can last for weeks. Orthostatic intolerance is significant problem for them. Matthew is resting heart rate sitting down 110 beats per minute. Alexander about 116. As examples, orthostatic intolerance they have tried --) and many others to no avail. Over the years alexander and Matthew have repeatedly said if there were one area of function they could improve, they would choose to be able to sustain cognitive function without the pay back of post exertional collapse. Earlier this month Matthew said to me he would choose cognitive function because what is made with the mind lasts longer. Thank you. (Applause) >> Sara Eggers: Thank you very much, Denise. I'll ask KIM McCleary, to next go. >> Thank you, Sara, thank you to the FDA for selecting ME CFS for first topic of series of patient-focused drug development initiative workshop.This is tremendous opportunity for our community and for advancing research and drug discovery. My comments will be based on the responses that we received over the last 35 days to a survey we launched on March 18, following the March 11 Federal register notice indicating that this would be the first of that series of meetings. We patterned our survey very much after the FDA questions that patients can submit answers to directly to the FDA docket. >> May you just say what association you are with. >> Yes, I'm sorry, president and C.E.O. of the association of America. We pattern the survey questions after those that the FDA published in the Federal register notice and we also augmented the questions with some additional questions that we felt were important in terms of setting the context for this conversation. Just to recap sort of the demographics of the population that responded to the survey, we had 1300 survey responses in 35 days. The average age of onset for patient symptoms was 32. And that broke down 250 people responded that they became ill at 18 years or younger so that's quite a significant pediatric population or population of pediatric onset. Between the ages of 19 to 35, there were 500 responses and again that is age of onset and that was the largest group of the four age groups. Ages 36 to 50 was 452 and then onset at age 51 were higher was 157. So the typical sort of bell curve. The average number of years since symptoms began at the time the respondent replied was 18 years of illness duration, this is quite a chronically ill population that responded to the survey and the range was within the first year they were responding to the survey all the way up to 70 years of illness. So we have quite a range. Our survey allowed patients to or participants, didn't have to be a patient, anyone who responded to the survey, although 86% of the survey respondents reported that they had been diagnosed with ME or CFS or ME/CFS by a healthcare professional. I think it is a good study population. They were able to fill in answers in open text format without any guidance or suggestion other than the same language that the FDA used. We conducted natural language processing analysis of the text that came in and used principle component analysis to design and to still down the answers. Out of 1300 responses, there were 70,000 words used in the responses, which then disstilled down to 970 concept IDs in unified medical language and these are some of the topics that came out most highly in the principle component analysis of the data on impact on daily life. What is the impact on of the illness on daily life. There were basically five areas that came out most strongly. The first was a fear of increased risk of death or disease. And that is not something that my fellow panelists have yet brought up. The second feature in terms of impact on life was that "life is not worth living or life stops," and this translated more into the loss of friends and vastly different professional life and that could mean either the loss of a career or career that was not the career that the individual was performing before the onset of illness. The third feature was the lack of effective treatments and this goes back to what was described earlier as unmet need. So this condition, the unmet need and lack of effective treatment has a major impact on people's every day lives. The fourth component was the following, I can't work because of sleeplessness, medication side effects, muscle pain exhaustion, blurred vision and migraines. So that answer actually responds to the question about symptoms and the 5th feature that popped up was social isolation that comes with the illness and that would be probably the hardest thing to restore with a drug therapy, but is again another major impact on patient lives. I don't have any more time, I'll stop there, but I have lots more comments I could give. We'll submit those to the docket. >> Sara Eggers: Thank you, KIM. (applause) >> Charlotte VON Salis, can you get the microphone to where you need it? >> I'm hoping, yes. >> Sara Eggers: Speak into the microphone. >> I can't move it. There we go. Okay. Better? Okay. 22 years ago I came down with myalgic encephalomyelitis, I meet ME criteria as well as Canadian consensus criteria and I'm always -- symptoms. With medical exception of 20% overall improvement that lasted two months while on antiviral prescribed due to low natural killer cell activity and high viral -- not had any significant remission. Most debilitating symptom fall under the rubric of neurological and cognitive dysfunction. I'm a lawyer, graduate of a top 10 law school used to reading, analyzing, writing and talking to judges and clients. Within days of getting sick, I could not read a memo I had written myself, had looked like hiro GLIFics on the page. I cannot think clearly, this includes inability to process information be it oral or written. I often cannot communicate properly, struggling to get a thought together, find the correct word and speak coherrently. I no longer able to read or write as I have before and have trouble understanding and remembering. My thinking has slowed down, acute eegrevealed predominant activity and have difficulty prioritizing. My IQ has dropped to 107. I also have significant problem with light sensitivity, you can see I'm wearing dark glasses and cannot focus too much visual STIMalation, for example, too many objects in a store, music and restaurant busy websites. I get dizzy, spatially diseriented, come down with headaches and feel nausiated in such environments. Extensive vestibular testing including computerized dynamic graphy confirms these symptoms. Sometimes a small amount of antiseizure medication helps, all too often I must lie down in a quiet room with the drapes drawn. I can't sustain any mental or physical activity for significant time without exertional exhaustion and exacerbation, of all symptoms, including fever and chills which last for days or weeks. Activity intolerance confirmed by cardiopulmonary exercise testing CTET. There are times I'm unable to do more than go to the bathroom or kitchen as needed. The impact of this illness is my life has been significant. I've had to give up my law career, I can't work due to my light and sound sensitivity and activity limitations I have to pay to have therapy delivered, laundry washd and my home clean. Socializing is difficult as I can't predict how I will feel at a given time and must avoid overly STIMulating environments. The later includes the internet, something many people with my disease turn to for social contact. I must limit my TV viewing and rarely able to listen to music. Phone calls must be limited, as well. I've had to give up one of my greatest pleasures, reading, relying instead on special audio book player that allows me to slow down the reader's voice and change the tone. I've moved to quieter neighborhood and adjusted to life that leaves me health bound and lying on couch or bed. I can't walk much more than a mile on a good day, had to endure -- balcony. I've lost almost half my life to this illness and still wait for appropriate treatment. I'm able to participate in this workshop only because I live five minutes from this location. Thank you. (applause) >> Sara Eggers: Thank you, Charlotte. So in order to make sure that we have enough time for the facilitated discussion, I'm going to ask my colleagues at FDA if they have any burning questions for any of the discussants up here? Dr. Sandra Kweder. KWEERD KWEERD Want to ask Mr. Lansen, you talked about confusion, I found that really intriguing and wanted to ask you, you said a few things I'm trying to tease out. Your description of being confused particularly your experience when trying to read something you wrote the day before. Do you have -- a sense of that cognitive confusion or more like a fog? Can you differentiate? >> It is difficult, but I can differentiate I think between cognitive confusion or fog, I think I've experienced both, I'm trying to sort out which I experience when. >> Okay, do you see words that you don't understand, is it like I don't know that word. >> Yes. >> Okay. >> For inSTAPS, I was just reading something about someone at the last name of Issue, but then it was a comment someone is here. >> Okay, yeah. >> Didn't get that. >> Okay. >> That is a strange experience. >> Yeah, I bet. Thank you. >> Sara Eggers: Yes, Terry. >> Dr. Michelle. Too many Teresas. I'd ask any of you to respond to this, but many of you have talked about the post-exertional malaise and I'll use that term for want of a better one. But you've all mentioned that this can occur both secondary to cognitive exercise or due to physical exercise. Can you differentiate, do you have different symptoms with your crash if you were exerting yourself mentally versus exerting yourself physically? And do you find that one to be a greater trigger than the other? >> Okay. I'll take that, I guess. Yeah. I think they are different. For me, the STIMalation of I think the cognitive actually having to mentally exert myself, talk to people or read or try to write or something, that does cause a crash that is somewhat different from the physical in the sense that my head feels like it's going to explode. Don't know how else to explain it than that. I really have to have total silence, total darkness. The physical exertion crash -- they both are the same in the sense I guess with mental, everything basically all my symptoms are exacerbatedthat, is why exhaustion isn't the correct word because yes, I am more tired than healthy people, but that is only one of my symptoms. It's basically exacerbattion of everything. >> If I could comment on that, John Kiser, one of the post exertional symptoms that is the strangest to me is post-exertional sore throat. So it can be physical exertion or it can be just working too many hours straight, within 24 hours I'll have a sore throat and it's not like I've become infected with a virus, it doesn't follow the normal course of 7 to 10-day viral illness, doesn't become strep throat, but it's more of inflammatory response and so I've learned that if I am in the midst it of exerting myself, either physically or at work for an extended period of time, I don't wait for the sore throat, I just take antiinflammatory to try and prevent it and that seems to help me somewhat, but I can always -- I can break through that, I can break through the antiinflammatories if either I go on too long or don't take them soon enough. So I wanted to make that comment. >> Let's move to engage everyone. If I can, Murphy's law will come into play. Come down into the front. I can speak closer to you. Terry, how much time do we get to have for this? Can we have a few minutes left over? >> You have 40 minutes and you have 10 minutes for each has -- >> We can go a little bit over? >> Yes, I've got about 10 minutes -- >> We have 40 minutes for this? Great. Okay, my first question that I want to -- do you have a question? >> I want to comment. >> I want to ask one general question first. And that is we heard five experiences and I thank you for those experiences. I know it can be difficult, it's difficult for me to stand up here and ask questions I'm sure very difficult to share your experiences. But what I want to know from the patients and those who have loved ones with the condition, how many of you related with some person's story up here, did you generally relate? If you feel comfortable, can you show a raise of hands? Okay. We're pretty reflective of experiences and at the end we'll talk a little about some of the differences that you might feel, but I want to focus first on the similarities. We'll go through some of the key symptoms and sets of symptoms that we heard today. We heard a lot about cognitive functioning, I'll use that as catch all. We heard a lot about the collapse. We heard about sensorial sensitivity to light and sound, we heard about problems with related to blood pressure and orthostatic intolerance, and then we heard a lot about the impact. Let's focus on the symptoms first. Start with the cognitive functioning ones, so we heard each of you, I believe, mention cognitive functioning. We heard brain clouds over, confusion and where you have significant time limitations. I would just like to see if anyone would like to build upon, share their experience about the cognitive functioning limitations that they would feel comfortable sharing? >> So we have -- I don't have to go to you, microphones will come to you. >> Okay. I have significant -- >> Can you state your name. >> Yeah. I'm Latina nicholson, I've had ME for seven years, significant cognitive dysfunction and partly termed minimally if I read something I can't -- short term memory loss, my body just shuts down. It's hard to concentrate. I can't focus. Talking is difficult. How to be quiet next panel otherwise I'll start stuttering or can't get words out. Those are most common, I have a lot more, but briefly. >> Can I just before we go to the next person, if you feel comfortable raising your hand, who has problems with finding words or getting words out and speaking? Okay. Okay. Okay. Any other type -- yes? >> Dr. Janet Smith, one of those working sick. And I find that decisionmaking is very stressful and I have more problems decisionmaking and getting to the question about whether mental fatigue versus physical fatigue, they're both extremely fatiguing, the mental fatigue I'm just totally wiped out, the physical fatigue has more muscle pain associated with it. >> Okay. Okay, so the challenges with decisionmaking or decisionmaking becomes harder, slower, more troubling, more anxiety about decisionmaking. If I feel comfortable raising your hand to say that you have that general experience? Okay. Okay. Anyone else? Okay. We're off on this side of the room, so let's go with Mary first. And then we'll come to you. >> I'm in the next panel, I will talk about me in the next panel. I want to talk about the people up here, I know almost all of you. I know many of the people in this room because I see you at meetings and what people here don't know, I do better because I'm on treatment. What people don't know, how much effort it took for these people to talk. And after people get up and testify one thing, you will hear them testify, cut off in three minutes or five minutes and they speak very slowly because it's hard to remember or read what it is that you are reading because you have cognitive dysfunction. I was very sick and testifying, I was through, I passed out on the floor and my friends knew what was happening, they saw me slipping from the chair. They got up and got me and brought me back and let me lie down in the back of the room and somebody brought me something to drink.This happened to a PREND of mine. I could tell she was about to crash, she got incoherrent and loud and boom, down she went. We had to take her in the bathroom and sit in the floor, get her something to drink, it took people quite sometime to get her settled. I ended up driving her home. She was incoherrent the whole way home, she had no memory of that, I had to spend the night in my house because obviously she couldn't drive. What a lot of you people don't know and we know because we take care of each other, carol in the back here, when I crashed once, carol and her husband took care of both me and I was with someone else who was a patient, we had come on the train. The patient, I had taken care of her and brought her, she didn't know how to get back on the train. So Ken, the husband had to take the other patient back to union station to get her on the train, she didn't know how to do it by herself. Carol took care of me and Ken came back and got me and carol. I laid down on the sofa while they called my husbands and he drove down to pick me up from Delaware. We met at BWY. The thing I want everybody to understand is that the patients who are speaking are generally speaking at great cost to what they will be like after this and I wish some of you could see what we are like afterward, what they are like, then you might understand this disease a lot better. Okay. >> Sara Eggers: Thank you, Mary. Before going back to the next person -- (applause) Let me just get a show of hands if you are comfortable, how many of you would put as a significant symptom for you the idea of confusion that is so bad that it scares you that you don't know what you have done, that you it causes anxiety. Okay. Okay. So we have I'm sorry, I can't see your name. Yes, in the -- Chris, thank you, Chris. >> I'm Chris Williams a patient and I'm going to be on a panel tomorrow. I just want to pick up on something that John was saying as someone working. I am no longer working, but I did work for 2-1/2 years after I got sick and I also had a very demanding job working in Federal government doing health policy and I was used to super vising about 70 people, running multiple activities etcetera. When I was diagnosed, I was diagnosed on the "mild" end of the spectrum. One of the things that I was no longer able to do and am no longer able to do is multi -task. And those of us who have demanding professional jobs understand what that means. Even though my husband has been very, very supportive over the almost five years I've been sick, he does not really get the no multi-tasking idea. When I tell him that I have to finish what I'm doing first before I can get on to the next thing, he really does not get that. Even though he lives with me and he's seen it. So I think that there is a sort of slippery slope going from what we were able to do as high functioning professionals to the impact of this illness. I also have another comment on something John said, could I -- you were talking about the crash and the physical versus cognitive. And I both as a professional and personally where somebody used to doing a lot of public speaking and one of the things while I fortunately don't have the most serious of the cognitive issues, when I speak, whether it's publicly or going to talk to my therapist about how depressed I am that I'm sick, I get a sore throat. And the sore throat comes on, I know it's going to come on, I know it's just a price I pay to be on the phone call, to be in a public venue or even at a social venue and I did not know about the antiinflammatory, but I'm going to follow-up on that. Thank you. >> Sara Eggers: Okay, we've heard a lot about cognitive limitations, first, anyone on this side have a comment? Any they want to share an experience? Just want to make sure I don't always look on this side. Yes. Yes. >> Tasha, I'll be talking later, I want to respond what I thought was an interesting question about the different way we react to mental versus physically. I have noticed a difference. When it's too much physical activity, I find that the sensation, the response is very much one of whole body throbbing, sort of sensation of some kind of inflammation, as well as muscle pain. With -- verse mentally, I get nauseous feeling with that and little bit strange to explain, sensation that a lot more focused in the head and neck. >> Sara Eggers: I will at this point ask if any FDA colleagues want to ask a follow-up question about anything they have heard so far? No? Okay. Okay. Does anyone have a real pressing cognitive one they want to talk about? Otherwise -- okay, we had a comment right there in the back. >> My name is Joan, a physician, I've had ME for 13 years. I'm not going to stand up because I really can't. Ide just like to comment that I think when you use the term anxiety that is not really correct. What people experience is fear, not anxiety, anxiety is something that you're worried about that might not happen, fear is when you know that something bad is going to happen and so you go back from doing whatever you you are doing to avoid it. It is important to make it clear this group of patients is not AENGS. We have a severe condition that causes huge impacts in our lives and we have a very legitimate fear of what happens when we overexert OURDZs, we have experienced it. >> Sara Eggers: Thank you for the clarification. (applause) >> Sara Eggers: I was going to say, I saw a lot of head nods and your claps reaffirm that. I know there are others who want to talk about cognitive, I want to make sure we move on to talk about the crashes that come on from the either the cognitive exertion or the physical exertion. If we have time, we'll come back and ask any other questions. We have talked a lot about what triggers it, what triggers those crashes is what I'll call them for lack of any other term. How many of you have heard when you heard other people talking like Tasha, and John talking say yes, I experience those similar to what I experience. Anyone who has something completely different that happens to them when a crash is about to happen they want to share? >> Times you have no idea when a crash will happen. >> Okay, can you elaborate please? >> Sure. >> If you could say your name for the -- >> There are times you have no idea when a crash will happen, something you normally undertake suddenly produces a collapse. >> Uh-huh. >> I just want to echo that, Joe Lansen, sorry. One thing could be the straw that breaks the camel back, you try to measure out your limited life as best you can and you overstep sometimes. >> Yeah, I agree, it's unpredictability of this that's really, really quite difficult to live with. >> Sara Eggers: Anyone want to share about follow-up on on build on what they are saying? Yes? Go ahead. >> Yeah, crashes are unpredictable for instance for me, I have to go two hours to see my doctor in New York, no one will treat me where I live in Delaware. One time just to drive 30 minutes to the train, get on the train, get off the train and then went to go get a cab, I actually blacked out and I started walking across the street and the bus started honking and thank God if it went from red to green, I just looked up, people were yelling and I was just wow. It's just happen any time. It's hard to manage that for me. >> Sara Eggers: Are there times when you -- ways that you have found that you can make it more predictable in a sense? Yes? >> If you could state your name. >> Hi, I am Dianne Mean, I'm a caretaker for my daughter LAURen, who is here with me. And I just want to make the point that obviously the symptoms that every one else has talked about are things she has experienced. She used to be also an honor student and athlete, got sick at age 15. One thing that we have noticed and that hasn't really been emphasized I think yet how interrelated they all are. So when you talk about the crash, you know, it's not just physical pain or not just head pain, it's also more cognitive impairment, more orthostatic intolerance, more neurological issues. It's they are very interrelated and I do think it is important to tease it apart, but we've been in search of the treatment that's going to sort of get at the core problem for the 15 years that she's been ill and without success. Because it's been our experience that when some of it is better, it's all better and if there is any predictability at all, the one thing that Lauren has noticed and this is more a negative predictability is that the worse sleep she gets, the worse she's going to feel during the day across the board in every way, the more light headed she will be and so sleep for her is something that we suspect is a major symptom. She has had two separate sleep studies which shows she gets zero (inaudible) sleep with sleep medication. >> Sara Eggers: Uh-huh. Okay. We have someone in the back. Sure, before we go there, Dr., would you like -- >> I'd like to know several people did mention and I'd like to see in the room for how many people lack of sleep or poor quality sleep is seems to be a trigger for crashes? Yeah. >> Thank you. Okay. Yes, let's go -- >> Hi. I'm Shannon cassidy, a patient, I just wanted to address the question of whether you can predict a crash and if there are any signs. I think there probably aren't in terms of a way I start feeling to say I'm going to crash. I think there are things I know will make me crash, which is pushing too hard, having been physically overexerted, having had to think real hard. I kind of know that it is going to come. Whereas other times I haven't done that and there is no warning. There is nothing I have done, there is nothing I can attribute it to and for me, literally it's an eye movement, like I'm looking here, I look over there and boom, I know I'm going to have a crash, I can feel my whole body changing. It's like an instant knowledge. >> Sara Eggers: How long will the crash last when you -- >> That crash can last anywhere from a day to weeks. >> Okay, thank you. We have a question. Yes. >> Just to clarify, when a crash sleeplessness connection, people were responding to the sleeplessness, but not the connection, can we ask the question again? >> Sure. >> The way you want to ask and make sure you get the right -- >> Right, I don't want to interrupt the flow here. I want my question is how many of you would say that when you know you've had poor sleep or when you had a poor night's sleep that that is likely to trigger a crash? Okay. >> Always had poor sleep. >> All right, but if it is particularly problematic, particularly because the mom said she knows when her daughter has a particularly difficult night that it's going to be a problem. >> Hi, Lee, I'm a patient and physician and I will be on the panel tomorrow. I want to comment about sleep. So there is always a concern that maybe people with CFS if they only slept better or longer number of hours they would feel better and I want to dispel that notion for me. I could sleep 10 to 12 hours a night and I do and don't feel good in the morning. Of course if I get less than 10 or 12 or have a bad night for whatever reason, I feel even worse. >> Right.That is what I'm asking. >> Sara Eggers: I see heads, is that generally shared? Okay. >> Hi, I'm Lauren, I want to clarify, it's not necessarily in the especially poor night sleep will make me crash, but if I've had especially poor night sleep and I do something that I would have normally done on a day when I got better sleep, I am much more likely to crash and I know that going in usually. >> Thank you. >> Sara Eggers: Okay. Just to stay on -- let's let Dr. Michelle ask a question. >> Actually two questions. One is by show of hands, if you could just tell me how many of you find that the onset of your crashes are always rapid? Like within minutes to an hour or so? >> So that is not necessarily the case tmay come on more slowly. The second question is with regard to duration of crashes, anyone could comment on if you have a more mild event that is set off your crash, are you likely to have a shorter duration of your crash? Whereas if you did more exertion prior to the crash will you have a longer crash? Anything predictable about that? >> Sara Eggers: I see heads nodding. Let's let Mrs. Melissa. >> For me, I don't know when I'm going to crash. I'm trying to remember what you just asked in the question. That is duration. The duration of the crash, no, it doesn't seem to correlate at all. And it may be that it was a lot of very little things over the preceding days or weeks that you're not aware of or just could be that you did too much in one day or had poor sleep. It's just sometimes it's there and sometimes it's not. When you feel good, you feel good. And you pay for it. We will all pay for this this weekend. But we PDP the chance to share it. >> Sara Eggers: Okay. Anything else about this and then I'll move on to another set of topics. Someone who hasn't -- Mr. Miller? >> So Robert Miller, patient, since 1982. So this is a great question because I literally got in yesterday and had a pretty good evening. Got up this morning, I've had a pretty good morning. I just went to the business center and my crash just started. So I don't know why I went to the business center, you know. I got there, sat down, logged into the computer and was like, why am I here. Now they'll be days when I can go outside, function with my kids and exert energy and will not have anything set me off, there will be no crash. So just like Pat just said, it's just like when it's coming, it comes. And sometimes it's not that you have done some major expenditure of energy. >> Generally I see a lot of head nodding in agreement with this. Before we move on, Dr. Burke? >> It would be good to sounds like there is many types of crashes, actually. I mean there are the kind of crash where you are blacking out, like crossing the road and blacking out and then there is the crash where you have a click and there is a loss of cognition or memory and I just think there is a lot that we could explore here. I don't think we could possibly get to the end of this in one afternoon, but I think that, yeah, if we could hear more about what we're defining as crash or collapse would be useful. >> Sara Eggers: Sure, can someone say, go with someone over there next to Mr. Miller. >> Dr. Janet Smith. There's two types of crashes, middle one Bob just described and that is actually what I sought treatment for, because I was finding myself going down a one way road the wrong way and over a bridge with no sight and could have been deadly. Another time I was in the hospital and I knew I had been in the hospital, but got off the elevator and couldn't remember where to go. The physical crash is like if you run a marathon and you bonk. I can barely put one foot in front of the other, I can barely lift my arm, I jerk. No, no, I jerk, too. So that's different crashes. >> Sara Eggers: When you say you jerk, can you just explain just so we all know what you are talking about? >> All of a sudden >> Your body is jerk something >> Yes, I almost knock the table over, my foot jerked and set the table over. >> Sara Eggers: Okay, is this -- >> Something more about the duration. >> And then the range of duration we're seeing that all the symptoms improve at once for some people and I heard one person say that it it could last for weeks or it could last a day and so can we qualify those crashes by time, length of time? >> The reason I'm waiting here, this is a basic question, I don't think has been well answered and I've reviewed studies on this, a few studies and what they find people can crash within a few minutes of an activity or even days after. And it is like a moving target N. Terms of duration, similarly, some people can crash and last a few days, but can last for weeks or months depending on the type of thing they were doing before. >> Sara Eggers: Okay, let's see, we have let's go with -- then we'll get amanda and get over here, okay.>> Hi, my name is Kathleen Harper, I'm a registered nurse and I've had several types of crashes, but the worst ones were after I had like minor skin surgery and pneumonia, after the pneumonia, I had a two-year crash, after the skin surgery I was -it was about six months and just this October I was -- I had to go, I went to the supermarket, I was feeling okay, I was with my daughter, which helps to be with someone, and we were -- they had no water, I needed to go, we had to go to another supermarket, well that did it, I was disoriented and everything, but I got home and got into bed. That night I had -- woke up with my heart beating out of my chest and I couldn't breathe and I called the ambulance and they said I was in atrial fibrillation and that was the first time that ever happened to me. So apparently pushed myself with my heart just is a muscle and it just went beserk and ever since then I've been trying to recover from that, where now I can't go to the store the way I used to, once or twice a week, I was able to do that. I just got DRICH here, I've been sick for 22 years and it all started with my teenage daughter getting mono, she's still sick and I'm still sick. And we really desperately need help. It's just getting worse, you know, now my heart is being affected I'm on medication that actually makes me more tired, so I'm trying to beg the doctors, give me something else, I'm like, you know, so sleepy, anyway, it's nice to be here and have a place to be heard. >> Sara Eggers: Thank you for the feedback. Should we let Mrs. Simpson talk and then we'll come over here. >> Right here in front. (Inaudible) my symptoms get to the point I can't go anymore, I don't have cognitive function left to focus to be able to speak coherrently or my body won't go anymore. I haven't had blackouts or anything like that, but they have -- they will last from a day to I've had one where I was in bed for 3-1/2 months and struggled to make it from my bed to the bathroom and yet here I am now. So it's just I think one of the most frustrating parts about the illness itself, you have no way to plan your life. It's definitely difficult. >> Sara Eggers: Thank you. And here. >> Karen heart, I'd like to speak to the crash, as well. I know where life's social envelope is, about one hour and 45 minutes. If I exceed that, I pay that price. Two weeks ago I had (inaudible) lady friends which is probably easy for you to do, but because I went to about two hours and 30 minutes, because someone else was driving, that was five days for me in the house. Because of extra 45 minutes of social interaction and that means five days of avoiding conversation, of not leaving my home, not answering the telephone, e-mail, all that stuff is just too much because of that 45 minutes. What is this going to cost us? Weeks. I don't have anything on my calendar for two weeks, because what is this going to cost, no way for me to predict that. >> Sara Eggers: Thank you. Yes? >> Go ahead. So there were three themes that came out that weren't necessarily due to words that popped out of our survey in answering this question and the three themes were restriction, dependency and adaptation and that is what I'm hearing a lot in the comments also and if I could read five quick quotes our direct quotes from the survey. My day is structured around my illness. Every aspect of my life has been adjusted, my job, my role as mother and wife. I call myself in jail prisoner of this disease. I have a very small life. I'm living a life of lowered expectation and I feel like this is a living death. >> Sara Eggers: Can I ask how many of you saw your own experiences reflected in those quotes? Okay. >> Yeah. I'd like to try and just put into medical terms what I think we're trying to describe about these crashs and that is it is almost like your nervous system at any given point just completely runs out of energy and you just fall off a cliff and it's then followed by sometimes an inflammatory cascade and you know, as someone who is recovered to a good extent from this, when I finish a work week, my Saturday and my Sunday are completely different experiences. I spend the entire day Saturday in the midst of recovery and fog and pain and just and exhaustion and fortunately that recharges my nervous system enough, so when I wake up Sunday I AUCH feel completely normal. And so for me the recharge occurs to a significant extent with a full day of bed rest, but I think for many people who are speaking who have the condition more serious at this point, that when your nervous system running out of gas, it can take weeks to recharge it. >> Or months. >> Sara Eggers: Okay, I want to do looking at the time, we have about three minutes three to five minutes left. I know that we aren't going to be able to cover everything, but what I wanted to get, see if we get to this. Is there anything that you have had in your experience that you haven't heard any one on the panel mention? You haven't heard it in any one else's comments? Okay. That you want to make sure is important to say we only have three minutes, I know everything is important, we'll try to get as many as we can. >> JA net and this is something that I think is very important and hasn't been brought up and that is my crash is pretty much correlate with very low (inaudible) function and high (inaudible) pretty much something that my doctor Dr. Peterson and I figured out. Function could be as low as one, no functioning cells at all.This is something miserable, not just subjective today kind of thing, we do have the science already to measure this and I think it's very important that people know about that. (Applause) >> Sara Eggers: Okay. Okay. Would anyone, any follow-up questions to that one? Okay. Any one -- okay, let's see, yes, we'll go there and go over -- >> I simply want to say I have blurred vision and double vision, do you have four eyes, that is disconcerting to look at. >> Sara Eggers: Okay. Mrs. Petillo. >> I am a patient and will be on the panel tomorrow. No one has mentioned gut problem, irritable bowel syndrome and other gut symptoms and how those flair up in reaction to activity and crashes and may have their own cycle even on good days your gut can be out of whack. >> Sara Eggers: Okay, follow-up questions about those problems? I think we have time for one more. Okay. Yes. We'll do two more, we haven't heard from you in the back there. >> So in the purple shirt, I can't -- sorry. >> Hi, joan, I want to comment that I think we're hearing varying description of single phenomenon and I would like to point out if we did not know that people with diabetes had high blood sugar and we just listen to them describe their experience when they have high blood sugar, we would have a similar variability in what we heard. So I want to make the point there is probably a single underlying thing that is happening to all of us patients that we have not yet identified. >> Very good point. And finally in the -- oh, yes, in the back there. Yes. >> Yes, I'm sorry, yes, raising your hand. >> Oh, okay. Hi, I'm susan victor, a mom of a patient and I just wanted to say there is one other measurable symptom that always comes along with a crash, which is the sore throat that has been mentioned and also a fever, so something is happening that is measurable in a certain sense. >> Sara Eggers: Okay. And I guess we have one more in the back. >> Hello, Anita, patton, 26 years. Two things, one if you are not sick you might understand like working on the computer and it says, not responding, that is like all of a sudden you have nothing to draw from. This is a scientific situation. Like there is always a why. Why is this happening? A lot of times this hasn't been mentioned, a trigger would be viral or bacterial infection, instant nothing. No output of energy. Output of energy is ATP, just basic science biology, whatever, high school crash where why is your body not making ATP. Those are like the questions that help us find answers. >> Sara Eggers: Okay, thank you. Okay. I want to sincerely thank you for your input into the first discussion on behalf of my colleagues up here at the table. We are going to take a 15-minute break so we will be back at 3:05. And at that time I would like for the people who have been identified for the second panel to work their way up at that break. Again, the restrooms are located behind us here and there are some chairs in the lobby and if there is any question, let me know, thank you very much. Oh, I'm sorry, one thing, Graf ham is telling me there are evaluation forms completely voluntary if you would like to fill them out and return them at the end of the day. (15-minute break) -- >> If you could take your seats getting ready for the second panel. (Inaudible) -- I'll get started with the second discussion topic. The format of the discussion topic will be identical to the last discussion topic, which I thought was truly such a good discussion and we look forward to the discussion to the next topic, which is really focusing on patient perspective on treatment approaches. And we have again five people who will present their comments first to set a good foundation for our broader discussion. We have Mary Demmic, Tasha, kellerman, Latina, Mary SWIETser, and amanda Simpson, they will share their experiences, they each have prepared a couple, two or three minutes of remarks, we'll go through their XHENTDs and ask follow-up questions for them -- of them and broaden it to the rest of the participants in the audience. Okay. The question hawe're looking at are focused on understanding the treatment approaches that you take as patients to help treat your condition or symptoms, including prescription medicine, over the counter products, nondrug therapies, such as activity limitation, etcetera, we're looking for what specific symptoms do your treatments address or how do you feel after you take those treatments. And then we're looking to see what are the down side of the treatments, for example the side effects. Now there are a number of treatments we're going to hear from up here and hear from our discussion and try to do exactly what we did for the first top and I can cover whatever we can knowing that the docket is available and we want to hear your full perspective through there. Okay. With that, I will turn it over to Mary Dimmock, to begin. Mary, (inaudible) -- In the years before he found ME specialist doctors were dismissive or gave ineffectual or harmful recommendation. One exercise at a gym landed him in bed for a couple days. After reaching ME specialist he was prescribed immunover, function and high inflammatory cytoKIEN, arthralgia inTOL RENS demonstrated by tilt table test. Medication to eleaveiate sleep (inaudible) supplement to treat low neurotransmitters and low court soland activity program to try to address post exertion malaise. He uses sleep hygiene, strict pacing and avoidance of sugar, alcohol and coffee and he was also on long rem men of treatment, antibiotics for lyme disease. Of all the treatments FLURnes, anti-fungal, the dietary avoidance and strict pace having had effect on specific symptoms or helping him avoid relapses. There are only two drugs that made a difference, provided a big difference. The arythromycin, for lyme led to increase in his ability to get around. He would be out of the house for five hours at a time. He was able to read books, he read 1000 pages in six weeks. He was able to walk a couple miles, but it only lasted six weeks, in spite of another year of IV, and oral antibiotic treatment it never came back. The second drug Kenra, for high i-01, it increases cytokines and normalized neurotransmitters, but produced limited functional change. He is slightly better, wakes up feeling slightly better in the morning. He's able to do about an hour of activity every other day instead of every fourth day. He's able to tolerate sitting up for longer period of time and has slight decrease in cognitive issues. We would barely notice it, healthy people in this room would not think it was important to them at all TOCHLT him it's like a miracle. It is not the dream he has of regaining his life. Because of his immune logical marker and lack of clinical effect doctors prescribed Rituximab, not approved for the disease, if he goes on it, he will have to pay out of pocket. He has access to one of the best doctors in the country, he has good insurance and well supported by his family and his wife. In spite of this over all this time he remains in bed sick with little quality of life and little hope that he will ever get better. He is willing to accept significant risk in new treatment and trying anything to give hem back his life and to escape the hellish debillity of the disease. Thank you. (applause) >> Sara Eggers: Thank you, Mary. I'll ask Cassidy to go next. >> My name is Tasha, kelerman, I developed CFS in 1986, following a tropical illness. I believe I can provide perspective on CFS since I've been diagnosed and treated for the illness in three different countries, Belgium uk, and the U.S. treatments have included everything from antibiotics, azirromicen, (inaudible) bencyclovir, injections and others. Exercise therapy, CVT and pacing. I'm currently on sickLOvir, and low doses of sebela, and amatriptaline. Other than the UK, I have been lucky to live in locations where I had access to CFS specialist covered through insurance. This is not the case for many patients. The main reason I wish to comment today is to caution against what I see as possible harmful trend to uncritically adopt the treatment approaches favored in the UK. I've provided background to my illness for context, my illness started suddenly in April 1996, when I was work nothing Angola, I developed sore muscle, fever, severe fatigue and lost approximately 20 pounds over two months. I had had a rash on my arms. I returned to Belgium and during the following years had recurrent bouts of throat infection with swollen gland, fever and muscle aches and developed headache and dizziness. I felt increasingly persistently fatigued. I found that my symptoms got worse after exercise. I was diagnosed with CFS in January 1999 and moved to work part time. I moved to the U.S. in 1999, during the follow it would go years my symptoms became cyclical. I would have four to eight week bouts of severe fatigue with sore throat, muscle aches, followed by relatively good periods FRCHLT October 2000 to may 2001, following testing revealing (inaudible) infection most likely contracted in Angola, I was treated withant biotics, I can't say for certain whether the treatment helped but I was consistently well until June 2003. I was able to work full time and to be fully active outside of work including doing physical activities. However, in June 2003, around three months after the birth of my daughter, I became very sick with CFS again. >> Sara Eggers: We can come back, we can come back and you can finish. >> I'll be fine, I'll hurry up. For the next few years I grew sick, worse than previously, I had to spend a whole year in bed and was mostly house bound for two years. I felt it was my great misfortune to be living in the uk, at this time. I am not offered any kind of treatment other than cbt program for which there was a two-year waiting period. I saw several general practitioners, primary care physicians who generally treated the illness with skepticism N. 2005, I was provided the opportunity to enroll in the pace trial in Oxford per which I could choose exercise therapy or cbt. I chose Get as it provided an opportunity to get physical therapy. I found the opportunity to see a physical therapist weekly to work on general stretches very helpful. I was able to increase the distance I could walk slightly, but remain very limited in my mobility. Get helped to a limited point. It was in no way a cure. The get program included a patient manual. The manual tried to explain how CFS symptoms, not including SICHLSoms like headache, dizziness, twitching muscle were the result of deconditioning. I was referred to cbtn2006, when my name came up on the waiting list. I went to a few sessions before leaving for the U.S. I did not find it helpful. By the end of 2006 I started to improve, I had started weekly b-12 injections in 2005 and think these did help with muscle pain and concentration. I moved back to the U.S. in 2007 and started on Valtrexnline with results of EBV and HERPes virus test. I've been on and off valtrex, and bansyclovir. I went back to work in 2007, starting part time and gradually been able to move to 25, 28, 32 and now 35 hours per week. I believe that the antiviral treatment hases really helped me. Of course it's hard for a patient to know what works for CFS because the symptoms themselves shift over time, they are much better today than I was for most of the past decade. Nothing I have tried is a cure. Of the many different treatments I've tried over the year, I believe the following have been particularly helpful at particular points in time. Valtrex, bite min b-12 injections and pacing. I don't see evidence for the others in my case. I believe CFS patients should be provided with access to trained physical therapist to work on stretching and exercises that meet their individual capacity. I believe this is a simple missing piece that could benefit many of us. For me, pacing is the number one most effective strategy without which I would never have been able to go back to work. I now have exciting job as executive director of small nonprofit organization. I still can't walk more than three blocks, can't stand more than a few minutes, but I'm lucky to have seen significant improvement in my mental concentration, I spend a lot of time in bed, need to rest after work and on the weekend, but being able to work has been very important to my health. I believe that the uk, does not apply the same criteria to the diagnosis of CFS that U.S. and other countries do. I don't remember being asked about symptoms outside of (inaudible) narrow definition of CFS outlined by the KAUND AUNDian criteria. I never had access to trained CFS specialist as claimed by the study offer. I think uk approach is harmful to patients. The idea that all CFS symptoms come from deconditioning that could simply be avoided by overcoming negative patent is ridiculous and offensive. The U.S. should think carefully and look a bit more closely at the Get and Cb pace trial before promoting in the U.S. (applause) >> Sara Eggers: Thank you, Tasha. Matina. >> Can you hear me? Matina, I've had myalgic encephalomyelitis for six years, prior to that, I was -- career senior management at top pharmaceutical company in marketing. Now due to the time treatment most significant that are most significant symptoms because my list of symptoms is very long. First, I have a lot of significant cognitive and neurological symptoms, but this time no treatment that are working for me. Or I need to really go back to a neurologist. Next is post exertialal malaise, I take just to it be here today high dose within label of ATAral, therefore I do that if I have meetings or advocate. Sometimes I take up to 60 milligrams of adarell, and I'm sleeping and listen to my body. For auto immune, I do cocktail, I can't get to my doctor two hours or three hours, I can only get that bi-monthly. I self inject get gamoglobin shot once a month, self inject b-12 and GLUT thighal. -- shot or pill. For sleep abnormity, I use AmbIE n, and Xanax. Both likely when I'm on Aderall, I do so much it is hard for my body to unwind. I kind of watch what I take, I don't like using something up and coming down, I'm very careful. So like last night I only got two hours of sleep. Pain due to Fibromyalgia, I use cynbalta, vicodin. (Inaudible) abnormities in disease progression, to date no treatment works well for me. They inject symptom control or symptom. By masking symptoms exasbait my symptoms and I crash, this could last several days, weeks or months. Currently I'm trying to get approval for Medicare to try IV ig. Otherwise due to expense I will not be able to afford it. Same with the other drugs in clinical trials. My current treatment don't improve my daily life.It allows me to get to an appointment or hopefully see a friend or family, but I never know, depends on that day. People have to be patient with me. For me any minimal mental or physical activity makes me very sick, leaving me prisoner to my bedroom. I have many abnormities with treatment. To date I have treatment option at this time in terms of low natural cells low growth hormone, high ebv, h-6, I tried all antivirals, they didn't work for me and one antiviral I use I broke out in hives. I have high interleukin, 6 and 2. I have bad cognitive dysfunction, I'd like to focus on research about cognitive rehabilitation therapy that is done for MS and lyme and lupus. Neurological issues balance, weakness of limb, twitchs, one leg drags, I don't know if it is neurotransmitter problem, sleep abnormity, you get refreshing sleep, so I think it is really need to do more sleep study in terms of CFS patients with and without (inaudible). Then nothing is working on my GI issue, I've tried everything. Most significant down side of treatment is use of (inaudible), I'm not talking bad about the drug, just for me, it's caused severe dry mouth, jitters, high pulse rate and I can't sleep at night. I need to take Ambien, only 10 milligrams because it makes me over 10 milligrams, I will drive and I don't know it, I will eat, wake up somewhere doing some weird things. 10 milligrams is my limit. This gets aderall gets me through the day today and hopefully tomorrow, I may look happy and healthy to you, but I'm suffering greatly, but I'm in survivor mood. Fortunate enough to attend this meeting while many patients cannot. At this time I'm mostly bed bound home bound 80% of the time. Suffering terribly with poor life and no hope I will ever get better. I'm willing to accept significant risk (inaudible) to live. I think I'm just waiting my turn for -- thank God for friends and incredible group of patients COMBCHLT CFS patients are incredibly strong and have a lot of courage. We look forward to partnering with you, the FDA, to find new ways to allow us to best track drug approval outlined by the drug approval process and fast track FDA care program for the disease and illness must be given at least equal consideration for drug development as HIV, (inaudible) complex serious debilitating disease I think this is a start. Thank you. >> Sara Eggers: Thank you very much. I'll ask Dr. Mary swiser. >> Hi, my name is Hairy Swiser, I've been (inaudible) 14 years. I was 44-year-old professor at Villanovain 1994 FRCHLT that point on I was very sick. I suffered from blackout ATA xia, disorientation, short term memory loss, dyslexia, massive confusion, I poured coffee into a silver ware drawer convinced it was a cup. Intense pain behind my eyes, I could not pass a (inaudible) envelope pacing treated hypo thyroidism (inaudible) didn't work and sleep with flex eril, and KRONAP im. Made me more comfortable, but my condition got worse and worse. My world grew smaller and smaller. By summer of 1996, I was falling every time I tried to walk, I had to get a wheel chair N. 1997, we added riser to the toilet and shower chair. I was confined to bed, only able to make it to the bathroom and back by holding on to walls and my golder retriever. By the end of 1998, I couldn't brush my own teeth. I was found positive for (inaudible) and found positive for 37 kta, defect, both predictors of success with (inaudible) my family decided to pay the expense was both in terms of money and time. I began (inaudible) on February 4, 1999 and two months I could walk without a cane, in five months I could drive again. For the first time in four and a half years, I did not feel sick. After six months effective (inaudible) gone and (inaudible) dormant. I read an entire book N. September I danced with my son at his weding and walked barefoot on the beach. It took care of symptoms described in this hand out. After that it became a matter of STAMina. It took longer, more years to work back up STAMina like a normal person. I continued to improve, but a year later, I stopped treatment, it is expensive. One year later I had a blackout and hVA 6 a was back. Took SECH months to bet GAK on the drug. We decided to stay on it and put the cost into our current income. I remain on the drug for five years before I lost it again. At that point I was doing so well I could go hiking for a half-hour with my brother N. January, 2008,let head of my practice died and we lost permission to continue receiving the drug. Seven months later I crashed again. I saw Dr. Peterson at lake Tahoe. I was running fever and had Epstein-Barr. My natural function was 3%. Abnormal speck span and cytokine (inaudible) permanent disability from a heart condition. In July 2009 spinal tap revealed my spinal fluid contained active (inaudible) as well as defective RNA. We concluded I had to get back on (inaudible) I moved to Nevada, 3000 miles away from my home and my husband of 25 years and started the drug -- excuse me. March 10, 2010. After two months I needed a cane. By summer I was walking along the lake and August my husband sent my car out to me because I was well enough to drive again. I had to spend over a year in Nevada separated from my husband. Then in the spring of 2011 I heard Dr. Would be starting treatment in New York City and I came home. Today I get (inaudible) by going to New York City twice a week, a 12-hour day, I take a local train to wilmington, I take Amtrak to New York and metro bus to the doctor, I take three hours, I get liter of iv saline that works for the nmh, and (inaudible) turn around the other direction, three hours and I'm home. It's that important that I remain on ampligen, without it, I would not be here talking to you, without it, I don't have a life. I can take care of myself, I have little STAMina, it takes longer to work the STAMina up, but my husband has aggressive form of cancer and he has to be cared for. I got five hours of sleep last night, we have a family crisis and my son and my daughter both turned out to be leaving on the same time tomorrow. I am stuck (inaudible) I can only get (inaudible) by moving away or leaving home 12 hours twice a week. Without it, I couldn't care for my family and my family would have to care -- have to do that, they would care for the two of us. Right now it's enough trying to take care of Bob. Please don't take (inaudible) away from me again, it is that important. Thank you. [ Applause ] >> Sara Eggers: Thank you very much, Mary. Finally, we have amanda Simpson. Can you reach the -- >> Yeah. Thank you. Hi, thank you so much for having me. I'm grateful for the opportunity and I'm so grateful to be able to be here today. A little over three years ago I had to put my 15 year old dogto sleep on Christmas eve, three weeks later my dad was diagnosed with cancer and the same night my husband was killed in a car accident on his way home from the gym. You can imagine it was a difficult and stressful time. And four and a half months later I woke up feeling like I had the flu. Two days later I was in the emergency room, 13 months, 30 doctors, across Dallas, Houston, L.A. and New York later I was finally diagnosed with ME CFS. My life had changed dramatically in 15 months. I simply refused to believe my life was over at the age of 44. And so I pursued the most aggressive treatment option I had available to me. I take 16 pills each day, I used to do well to remember to take my vitamin. I take two injections each week. I've been on 100 milligrams of sebella two times a day since August of 2010 for pain management and increased energy levels and quite simply I believe it's the only reason I've been able to remain on my own. And that I haven't been completely bedridden. I also use Tylenol and hydro codon't for pain management, I take (inaudible) to help with my heart rate and blood pressure, both of which were fine before I got sick. It took a while to find the right sleep medication, I now take (inaudible) 12 and a half milligrams and can usually get seven to eight hours of sleep a night TOCHLT help bolster my immune symptom I take (inaudible) injection from Dr. Inlander, multi vitamin, fish oil, vitamin C and D, supplements. In October of last year I began taking GSMath and I've had significant decrease in the number of symptoms including weakness, pain, brain fog, sore throat, nausea and sensitivity to light and sound and experienced significant increase in energy and functionality. My EBV, hhv6 level have decreased. I've embraced helpful nondrug therapy including acupuncture and choiro PRAKTic care to treat headache, sleep disturbance and pain. I believe they have made a significant difference for me. I changed my diet, eliminated almost all processed food, I drink at least 80 to 100 ounces of water a day. I live (inaudible) from my diet and I'm in the process of converting over to the super immunity diet by Dr. Ferman. I use (inaudible) to help with my memory and concentration issues. I try to play that every day. I rely heavily on my faith, my family, my friends and my two sweet dogs to help me maintain a positive attitude and I think depression is maybe one of the things that such a misunderstanding about this disease, you know, it's easy when you feel like your life is over to because of the way you feel, but I think that the positive attitude and believing that somewhere some day out there there is hope, has helped a whole lot. And I also severely limit my stress and activity levels. Down side to all this, the drugs have competing side effects, sabella gives me energy, (inaudible) makes me tired. I take drugs to help sleep and it goes on and on. There are more humiliating side effects, of personal nature and I won't go there. But finally, there is the cost, my medical care can cost as much as $2500 a month. It was really important to me after those initial 15 months to find way to still matter. It's hard to do that when you are couped up in a bedroom. I can say, although I'm not as well as I'd like to be, these treatments have restored a great deal of purpose and meaning to my life and so I'm in the process of trying to start a nonprofit organization to help speak up for those with this illness who can't speak for themselves. >> Sara Eggers: Thank you. [ Applause ] >> Sara Eggers: Thank you to all the panelists. I want to see now, I don't want to limit the time for the broad discussion so we'll bring you guys back in. Before I go to the broad discussion, I just want to see if any of my colleagues at FDA have any burning questions that they want to ask as follow-up to the panelists? Okay. Let's go down and I did this to myself again. It could make a loud noise. See if I can do the same thing. Thank you for sharing your experiences and hope we can build on the experiences. I want to ask the same question I asked at the start of the last discussion, which is how many of you saw your experiences reflected in at least one of the panel members who spoke today? Okay. Okay. I know there are differences and at the end we'll try to get into the differences again. But for now, I'd like to focus on the general types of treatment approaches that we heard and get a little build-up more on what we heard from the panel members. So we heard a lot about treatments that are an attempt to treat the underlying source of the condition, the immune mod laters immunosuppressants and the antimicrobial, antibiotics and antivirals. I'd like to spend time on that. We'll spend let's see how much, we have -- Terry, can you just -- 4:15. Okay. So we'll spend some time on that and move on to the ones that really are our targeted to treat symptoms that are more symptomatic components. Okay. So would anyone like to start? We heard about ampligen, rituxin, and antimicrobials, would anyone like to build on what they have heard? Let's start with ampligen, anyone want to build on what they heard? Yes. >> JA net, I want to make quick, I had a treadmill stress test about three weeks ago and I had two horrible days after that, typical symptom flare ups, didn't sleep, my digestive system was messed up, pain, it was pretty horrible. Two days later I went into the office to get Ampligen infusion, barely made it to the office. After the infusion I was bouncing around the office, it was immediate huge improvement. I also have last 2-1/2 weeks gone through two moves. I live in (inaudible) like Mary was and my daughter and husband live in the Bay area. We happen to have two moves and I've gotten through them and here I am today. And this would not have been possible without Ampligen, I'm more functional because of the drug, it's like I'm a new person. >> Sara Eggers: Can I ask, every one takes different treatments, so if you feel comfortable raising your hand that you have had an experience like what JA net has shared with this sort of instant -- okay, we've got -okay. Some in the back, okay. How about sharing experiences like what Mary talked about. Okay. Anyone else like to add upon this? Okay. We'll go with both of you. >> Janet Smith South Dakota. In 1998, I was diagnosed with IGT deficiency and started on gamaglobulin, it helpeded for three years, it was then either retire, or disability and do something drastic, I have been commuting to the village from South Dakota weekly to get Ampligen, at first I couldn't walk up the jet way. The first time I tried to blow out a candle after I started Ampligen, the wax went all over the table. I've been commuting now every other week because I'm starting a practice out in Nevada, I have two practices going and I wouldn't be here today if it wasn't forAsmligen. >> Sara Eggers: Okay, Mr. Miller. >> Robert Miller, patient since 1982. I want to build on on my previous statement of my crash has started. Because of Ampligen, I will bounce back. I've already started to feel better just from sitting and is resting, but had I not been on Ampligen for this trip, I would not have made it here. Before Ampligen, I was literally bed bound. When people talk about being bed bound, we are like bricks, we can't be moved. My wife would come and check on me to see if I was breathing because I would sleep for days at a time. I didn't get up to eat, didn't get up to go to the restroom. My Dr. Dr. Peterson enrolled me into the Ampligen clinical trial in 1998. At the end of that hemispheric the drug sponsor gave everybody six months of drug for free. Within that span of time it was like, hard to describe when people ask you about your energy level and cognition, for me it was like little glimmer of light that I could feel and see again. And I kind of built off of that. Prior to Ampligen, I had been placed on on Samvir, Zantrax, ZITHromax, amoxicillin, proVA c, veseral, other depressants, lerica, did acupuncture, when we're talking about symptoms, it's difficult for us to say that and JA net did a great job earlier of explaining that when my energy level is low and I'm tested, my natural killer cell numbers will be down to 0. My natural killer cell function will be down, prior to Ampligen, t-cell count was 285 O. Ampligen, it's five times that. What Mary described is very much a mirror of what I've experienced. I've been on and off ampligen, I did can so well on it and my wife had a chance to move from the reno area to DC for a promotion, that we talked about Dr. Peterson, my health was doing pretty well, we talked about what the percentages would be if I went off Ampligen, whether I would maintain health or slide back. It was about 66% chance that I would at least maintain it. We moved to northern Virginia, I was there two years and went into a slide and went right back to where I was when I very first started. So in the midst of housing crisis, we sold everything we owned, we moved back to reno, Nevada, so I could be close to Dr. Peterson and get back on ampligen, it's different for every patient like every medication is. It's not a cure, I am one of the most severe type patients and you can certainly confirm that with Dr. Peterson and for any medication to get me up and allow me to think clearly, allow me to function, allow me to make my own meals, allow me to go outside with my boys, is a miracle drug. Thank you. >> Sara Eggers: Uh-huh. Thank you. I want to make sure we touch the range and I think if you agree that you are experiences on that particular drug are reflected and shared by the experiences we just heard, then we'll move on and talk about other treatments you are taking to really try to deal with the underlying condition. So -- >> Hi, my name is Lilly. A lot of the treatments that people are talking about other than symptomatic treatments, I think are limited to a very small population of CFS patients. I see Dr. CLIENmas and montoya, I have enough insurance, money and supportive family to help me get to those places and a lot of patients don't. This is a selective group able to come here. [ Applause ] >> Sara Eggers: Uh-huh. Okay. Yes? >> Hi. I'm carol olsen, I've been sick for 28 years, getting sick in Denver when there was an outbreak. I've been many things many of you have done. One thing that's most significant to me is no one has mentioned was (inaudible) with Dr. Peterson and that is also (inaudible) is very potent Aids drug thad tremendous effects. I look differently, I lost a lot of weight, my weight had been going up and down, I had always been kind of thin, but I wasn't. And that seems to happen to a lot of people. But most important thing was that I could think quite clearly. And at any rate I don't respond well to toxic medicines, this was a toxic medicine which most hiv and aids doctors will tell you and a lot of them have had experience with it. It also seems to treat viruses nothing else touches. I think in fairness and another reason you can also see this is biological disease that has physical causes when something affects it so profoundly. So I think both for that reason and for the reason that it helped many people significantly and I believe Dr. Peterson just had a study with it, I think that something else you ought to throw out. I'm also doing gc Moss now and I think it's helpful and I'm not really sure, but this diet is something that had a powerful effect with me for seven months. Thank you. >> Sara Eggers: Thank you COMBCHLT questions? Oh. >> This is just my opinion, I think it's great, you know, these drugs are not going to work in all people, so we have to look at that. Because I think of it as cancer, if you have CHEEMotherapy, it will not cure all cancer, you have smaller brain cancer, one type of chemo therapy is not going to help different type of cancer cell, so it is great to see like a lot of these drugs that are working on people. We need to put that into some type of measurement because we are unique to allow these people to have drugs that work for them, that work in subset of group versus use case definition such as bercuda, is that right? No drug is going to have the efficacy for that entire group. >> Sara Eggers: Yes. Okay. Anyone else want to talk about we haven't -- let's see. Both Mary and tasha talked about azythromycin, anybody want to share anything with that okay. I'm going to look to Dr. Michelle and see if there are other treatments that you would like us to follow-up on? >> I'd like to move to the pain management category and if folks could talk about their insurances with some of the products approved for Fibromyalgia, I think I heard a couple of them, people mention Sabella, and cynbalta. >> Sara Eggers: Okay, yes. >> Karen HART, good luck with this compounded formula LDN, I just started on that and it's very nice. >> Sara Eggers: Anyone else? >> I have a question. >> Sara Eggers: Yes. >> Cymbalta, works well, I tried Lyreca, the drugs have different side effects that work differently on each person so basically it's either like antidepressant, work for you or MOT and you can try a different antidepressant in the same class SRI in my opinion. You have to find a pain killer that works for you, I don't want to talk bad about it, it just didn't work for me. >> Sara Eggers: How long would you try something before you would decide that wasn't going to work for you? >> One drug I don't want to mention, I knew in the first two days because I had significant serious side effect. I with Cynbalta, it is antidepressant, usually takes two to three months, I give it like two or three months, depending on if it has significant pain, shorter, but I try to allow the drug to work based on whatever the indication says in the prescribing information. >> Sara Eggers: Okay. Is this resonating? This discussion about pain and pain management resonating? Okay. We'll go with -- yes. >> Jill. Yes, jill anson, cymbalta has marginal pain management benefit for me, but had side effects including intermittent loss of vision. I lost access when I lost insurance, I'm M the VA health system which does generic, nothing on patent. Cymbalta should be off patent shortly, we may see it in the VA pharmaceutical (inaudible) who knows. Thank you. >> Sara Eggers: Yes. >> Jenny petilla. I've been on pretty much every antidepressant for pain management and not a single one has worked so we had to go to codeine fixture lexeril, gobopensin, which no one has mentioned yet and the time period for trying a drug, I'll give it eight weeks at least before abandoning it and over time I've been sick 18 years, the pain management process has evolved substantially during that time. I probably tried at least 25 drugs, maybe more and over time they lose effectiveness, I have to switch it up. It's probably the biggest part of my treatment regimen, managing the pain. >> Sara Eggers: I see a lot of head nods. Your experiences match what Jenny is saying? Okay. >> Yes, Amanda. >> I want to say, when my (inaudible) hasn't worked and I needed to take pain medication, one of the most frustrating things, once you get out of that realm and into the kind of things you're talking about, for me it does nothing but contribute to my brain fog and the memory and concentration, it makes that so much worse and make its harder to come out because you have got all of the narcotics in your system and that for me is frustrated. >> Sara Eggers: Could I follow-up and say how do you make those choices? How do you weigh the benefit that you think you will get with one drug with the down side you think you might get and stay with this example you are talking about, the pain management versus the brain fog. >> I have gotten to the point where the only time I will take the pain medication beyond the cabellan it is basically I'm laying in bed and can't move because I hurt that badly. If I can't sleep, can't find a comfortable position to rest in to the point where I just can't function or bear the pain it is most important. >> Sara Eggers: Is this a shared experience? There is the last hope when the pain is so bad? Okay. >> Also ask people like a lot of us have major stomach issues and we have to really watch what pain management we take it is like give or take, so I want to see what people have done with that and also I think I want to reach out to researchers to look at medical marijuana because a lot of good studies effective and work on sleep, as well as pain, it works on neurotransmitters, that is something. >> Sara Eggers: Anyone want to take managing side effects intestinal? Yes? >> I have serious gastric issues and most of the pain medicines have basis there Nsaids, can't do them. I use volterin GEL on my joints, I don't whether it works or rubbing it in use, I have a Tins unit and just deadens the nerves and I do that when I'm laying M bed and can't sleep because it hurts so much. >> Can I say something? I want to mention you talked about in terms of Fibromyalgia. Not everybody with this disease has Fibromyalgia and they are not the same thing. There is an unusually large number of people who have both, but at least half of us don't and probably more than half that doesn't mean I don't have pain issues, I do, partly because I gained a lot, I was in really good shape before I got sick. Six months after I got sick, my metabolism was affected and I gained weight. Part of the problem, I fell so many times. You learn you can't walk because you fall. I've fallen so many times, I've had three back surgeries, knee surgery, knee replacement, I have pain issues, but it is not Fibromyalgia. I have muscle pain issues, it is not Fibromyalgia either. There is other, irony is when you talk about bad side effects, one drug I can't take anything with tilenol in it. It makes my liver markers sky rocket, which is ironic, of all the other drugs I'm taking, but I tried cymbaltancombination with antibiotic I ended up passing out (inaudible) we don't all have Fibromyalgia. When you talk about pain and people with the condition, it's not necessarily Fibromyalgia. >> I might clarify, my question was not meant to imply people with Chronic Fatigue Syndrome all have Fibromyalgia. That is not what I was getting at, I was grouping the medications together since they all have an indication for Fibromyalgia and pain specifically related to that. >> Sara Eggers: Let's go, we haven't heard -- >> I just wanted to say since this illness is so long-term, which we didn't know in the beginning, but my daughter was 14 when she got ill and by 18 doctors had put her on oxycodonePercocet, and she had to take a lot of Advil, and wound up with eSOF GEal ulcers after a few years of that and would have, she needs to go into rehab or something to get off these medications, so I mean, I am very angry that she was ever put on them. They wouldn't let me in on the decision when I called the doctor and said why, questioned, they -- what they were doing, they just told me I had no right that she was an adult. I'm her caretaker, she has to live with me because she's completely disabled, couldn't even be here, and it's just a nightmare. 22 years of pain medication does a lot of bad things and I'm getting older, I'm in my 60s now. I don't want to take anything that is going to affect my cognition, which is already, you know, poor from the brain fog. >> Sara Eggers: You are making a good point, I want to make sure we are capturing up here is that I think what I'm hearing is that since the diagnosis wasn't made, wasn't easily made, that you were trying, you and your daughter were trying a number of other things and in the end if you knew what you knew now you wouldn't have tried that. >> If I knew 22 years -- >> Sara Eggers: Is this a shared experience with everyone? I see a lot of head nodding. Yeah. >> Like I said, she had hemorrhaging, esopHageal ulcers at 30 and her pulse is now, she doesn't just have Chronic Fatigue Syndrome or ME, she has ulcers and you know, just it's a nightmare, it's a nightmare, I'm sorry. >> Sara Eggers: No, if I can take, we'll go with -- >> I just wanted to add to Mary's point earlier, besides Fibromyalgia, different types of pain in CFS. The definition includes muscle and joint pain, there haven't been a lot of research in to different pain in CFS. For example, some people have nerve pain, that is related to like react VAGZ of HERPes virus and some people reported they get better when they take antiviral for that. There is stomach pain, you know, other types of pain. >> Sara Eggers: Dianne, right? She had her hand up. >> I'm not on any pain medication at this time, although I certainly would like to have some. But one thing that I I'm a social worker, not a doctor, but I attribute a lot of my pain to the fact I'm not getting enough oxygen and therefore my muscles are really hurting and so you know, my lack of ability to deep breathe, you know, my sleep issues, there's a lot of I think a lot of reasons that cause the body to be depleted of oxygen and just no way if a doctor doesn't believe that there is anything wrong with you. I am on an extreme amount, 400 dollars a month in copays for medications and none of it is working. It's all palliative care, it doesn't get to the core of the problem. Ant virals, pain medication, a lot of medications here, nobody has mentioned proVIDual, it keeps me alert and it does really help and I also use VyVA nse, instead of aderall. As far as the pain goes, I think a lot of pain comes from lack of oxygen and your body is just not -- nothing there for it to draw on. >> Sara Eggers: Can I, I'm going to ask one question if we have time. I caught up on something and I didn't write down who said it. I would like to probe a bit deeper into the idea of masking symptoms and causing by taking one medication to mask symptoms, I think I'm sorry, I don't know which one, the idea that you maybe push yourself a little bit more than you should and you would have if you weren't on the medication that make you feel good right now. Is that an idea -- >> I thought about that for a while. I think when I'm on it and I can't speak in terms of the pharmocology, I just think my body is on overdrive, even if I try to even pace myself, very hard to pace because you are full of jitters, I find myself feel my body running when I'm siting and not turning off. I feel like I'm a car and the wheels are turning and I'm not moving and that is if I'm lay nothing bed, that is just my side effect. She mentioned Providual, I tried it, it made me jump out of my skin. I think different people respond to different medications, you have to find one that works for you. In terms of what you said, usually because of medication, it lasts in my body longer and (inaudible) not for me. >> One comment, too. It's not actually related to your question. My own personal perspective and not to go contrary to the purpose of this workshop, we need medicines to help people with CFS and but my philosophy is to try to take as few medicines as possible and I can do that maybe because I had the illness for 16 years and have a good sense, I've tried things and they haven't worked, I discontinue them. I think we really as patients I hear people are taking a lot of medicine and probably a lot isn't working. >> Sara Eggers: I think the doctor has a follow-up to that. >> Yeah, you mentioned that you thought one of the adjunctive treatments that helped you most was physical therapy and the stretching. Can you say a little more about what it was that helped? How did it help you specifically? >> Yeah, I only did that for a short while and I have to say that was really probably the only thing that was good about the treatment that I had in the UK. I think it was having a trained professional who knew through conversations with me what the limits were, basically at that time I could really only do lieing and sitting exercises. But was able within that framework to give me very limited exercises that I could do every day and I think having that guide and none of us are specialists in this and often don't know what to do. We want to increase our capacity as much as we can and we just don't know the best way to go about it. I mean the other thing I would say going back to pacing and I disagree with some of what just from my own experience others have said, I feel like I can tell when I'm overdoing things, when I'm getting to a point where it is going to be too much. My life is on a daily basis a very carefully collaborated system, you know. I have a mat, I lie on at work. I've arranged all kind of complicated work arrangements, I don't work a full day from the office. I work from home much of the time. I think we really have to figure out kind of by monitoring ourselves carefully what works and didn't work. Of course some of us, I have a wonderful husband who is there to help is and some people don't have that kind of care and help at home. I think if we really can do things ourselves that will help. >> Sara Eggers: Would anyone, let me see if anyone wants to follow-up on that and build on that? >> Okay. Thank you. >> Yeah, just quickly to build on what Tasha was saying I maintain a yoga practice and which is kind of California physical therapy, I mean, what is stretching, what daily or every other day stretching does, releases endorphins and so there's a natural release of natural pain control that comes from developing a practice like that and when you first start you can barely do a stretch or a posture but that is why I think Tasha's point having access to a trained professional, they can help you build and develop that so that it can have some effect. >> Sara Eggers: Thank you. I will turn and see if my FDA colleagues have other questions about particular treatment approaches, things you haven't heard of about that you want to probe a bit deeper? Dr. Michelle. >> One thing that has been mentioned only in passing, but has come up in the course of discussion of clinical trials is IV saline I'm wondering how many of you have used that and if you find it to be beneficial? >> IV saline. >> Let's go, next Dr. Kiser. >> I'll tell you the abbreviated story. I flew down to Puerto Rico with my grand solicit son, who didn't have the right paper to get on the cruise ship and after many stressful hours we finally got him on and I had a total crash. The next morning when I woke up I said, I've got to go to the infirmery, I went in, my blood pressure went down to like 65 and I fainted. The Belgian doctor who understood what Chronic Fatigue Syndrome said I can either send you home or give you a bag of saline. He gave me the saline drip, I got up and went shopping. [ Applause ] >> Sara Eggers: One in the -- one of you in the back. Did you want to -- add to that? No? Okay. Come back up. Someone else back there? >> Yeah, IV saline helps, I consider it a treatment. Relatively cheap one. It helps with the light headedness and I also tend to get a little bit dehydrated when I have a crash and obviously helps with that. >> Sara Eggers: Yes? >> My name is Joan, I would like to say you can also use oral salt and water that can be very effective in an emergency. >> Sara Eggers: Any other burning questions? We have five minutes left and I just want to do what we did in topic for the first topic which is see does anyone else have anything they want to share perspective or something to share they haven't heard shared by the comments from the panel or by others? Okay. >> Joan again. I'd like to just say that in terms of pain management, if somebody has a broken leg, it will be very painful and it will be less painful as the leg heals. If you break that leg repeatedly, it will get more and more difficult to control that pain. So we have the equivalent situation here. We don't know what the underlying cause of the pain is and so therefore we cannot probably adequately treat many people's pain. So I know that this is an FDA meeting, but I know there are members of other agencies in the government here and I would like to put in a big pitch for the fact that we need more research, more money to look at the underlying causes of this illness until we find that people are going to be taking drugs, having side effects from the drugs, watching the drugs effects dissipate over time because we are not treating the disease. I'm not saying we should not treat symptoms because we should, we need to make everybody comfortable and we need clinical trials period of measures, but what we really need is research. [ Applause ] >> Sara Eggers: Here and then -- >> I have a topical pain medication, so therefore it doesn't really affect my cognitive abilities, thank God. But what is in it, (inaudible) lido cane, cycle, baclofan, I don't know how that is pronounced, anybody is interested in percentages, they can see me at the compounded prescription. It works beautifully. >> Hello, Anita Patton. Will you please ask question of the audience like you ask us to raise our hand, will you ask how many people represented here today are from any sort of pharmaceutical company that could help us in drug development? Do those -would anyone care to volunteer themselves as being from there? [ Applause ] >> Any other final thoughts? I think we have time for two more people. >> I just want to -- obviously what we really need is approved drug treatment and that is why we're all here and I think that's the most important thing. I want to give big shoutout to the research of Stacy Stevens and Chris Snell, I wear this heart wave monitor all the time. I know what my threshold is and I don't want to go over, I will crash. I brush my teeth, I sit down so I won't go over.That is valuable and I think that is something that is maybe underutilized at this point. >> Sara Eggers: Okay. Okay. >> One more and then we'll come with your question. Yes. >> One other thing that -- Janet Smith. One other thing that hasn't been mentioned that goes along with physical therapy and heart rate monitor is I've always asked myself why am I better than a lot of patients as far as physically and I think it's because the adaptation has come naturally for me. Like the shower chair sitting down in the shower, I sit down whenever there is a chair available. So I have a handicap sticker, since 1994, and so if by using the handicap sticker I can do one more stop at the store or see one more patient because I'm using those aids, that come naturally for me, but other people they don't think about sitting down and how to reserve energy. >> Sara Eggers: Thank you. We have one more question from Dr. KweedRESHGS. >> It's a comment, I want to pick up on something that Joan said, which is someone said it and might have been you, Joan, in the last session about getting at the underlying cause. And I don't want people to think that hearing about the symptoms and the varied experiences of patients, even if it is common underlying cause and everyone experiences it differently, is not a worthwhile endeavor and I do think it is worthwhile in that it helps us develop pathway research pathways to explore where we see some of the commonality, where we get hints about potentially some of the common patho physiology that those particular agents that are helpful are addressing. Our goal is to -- that is one reason why we want another researcher here and one of our goals is to stimulate more conversation, more people smarter than me thinking about how could we tie this all together pathophysiologically and really begin to target therapy development so yeah, we're helping symptoms, which may be first on our plate, but ultimately getting to the bottom of this and understanding what that common root is so that we can actually cure it. So your point absolutely well taken and we are hoping ultimately this kind of exercise will is one way to contribute to the building the map. >> Sara Eggers: Thank you. [ Applause ] >> Sara Eggers: I think that is a good place to stop with the discussion 2. Again, a fabulous discussion and there are -- excuse me, I made it this long. There are evaluation forms and if you can't find them, I think they're in the back, come find me or one of my colleagues and we'll find them. They are completely voluntary, if you want to take one in and if you want to take it tonight and think about it, we'll be here tomorrow. With that, I'm going to turn over to Theresa Toigo who is going to close with the open public comment session. I will ask the panel members up here, you are free to go down, thank you again, so much. [ Applause ] >> Theresa Toigo: I hope the panel will indulge me and change our plan a little bit because it seems like we can maybe do a lot of this from people sitting if they don't want to get up. So we'll try something a little different here.This is the open public comment session and both FDA and believe believe in transparent process for information gathering so we need to do a little formality here to ensure that we take care of the process the requirements. We're going to invite the stake holders that are preregisterd and confirmed to share their perspective with us during this part of the meeting to to ensure the transparency tis important to understand the context of an individual's presentation, so you are encouraged to disclose at the beginning whether or not any organization paid for your expenses to attend this meeting and if you choose -- that is fine, as well, that is important part of our open public process that we explain that. So how is this session going to work? We had originally intended to use a timer, but approximate you are willing, if the speakers are willing to work with me, we have two minutes for each person to speak and instead of timer, I've got my timer and if you are willing, as I'm going to watch it, as you are getting close to two minutes I'll let you know, I'll put my hand up and then that way we can ensure that we get to everybody that everybody is able to get their allotted time and that everybody else gets out of here at the time they want. But I think we've heard enough today it's not easy for people to stand. So to make you come to the center to do it in the timed process is probably not ideal. Are you all willing to work with me who will be speaking in open public session? When I go like this, know you have to get close to wrapping it up. Okay. That is how we'll do it. So I think what is important here is that we know that not all patients speak with one voice and we certainly heard that today. People are at different stages of a disease and therefore things that matter to them are different. But the insight you can provide us are going to be helpful to the sessions tomorrow and they're also important as we think about the challenging issues related to drug development in this particular disease area. And so we want like I said to be fair, so you're willing to work with me and I'm willing to change the system and hopefully my panel here is going to allow me that, so give me some daggers if I let the time go to go, let's give it a try. Our first speaker it's not on there yet. Okay, the first speaker I have on the list is Mrs. Courtney alexander. Mrs. Alexander here? Okay. So Mr. Michael walser. Okay. How about Mrs. ANATa patton? Okay. Anita, do you want to come up to the microphone? Okay. >> Steven is next, if you are going, so you are ready. >> Hello, thank you, a lot of cords over there. Thank you for having this drug development meeting. I think it is incredibly valuable and a pleasure to be here. I would say so similar so many things about what so many patients have talked about today, except that it naturally falls into several different subsets like some people have sudden onset, slow onset, some people have low n-k cells, high cytokines, those type of things, doctors could get together, just an idea, get together subset and really put their research, their science, their experience with patients and over like some of the decades to identify which people might most likely respond to much treatments and my question about the pharmaceutical companies is how can we draw them in if there is not a whole lot of representation here today, how can we get that and how can we facilitate faster treatments to help more people? To that ponent I would bet there are a lot watching through webcast, they're certainly probably hearing the input and some of them here. >> The last thing of course I'm ampligen responder, 15 years, long time on it and I'm disappointed that it wasn't approved and hoping like is there any way to put some sort of another trial or another type of investigation to say that the people who did respond ydid they? How can we help hundreds or thousands of people who could have a response if there was some sort of global trial, another one that would be done. >> Okay, thank you for the comments. The point of this is not to respond, this is open public session, we're taking comments. >> Thank you. >> So next on our list, thank you, is Steven chillinski. Courtney alexander. I'm sorry. I have a different list here. >> Yes, it's been revised three times. Courtney, go ahead. >> I'm not courtney, I'm standing in for courtney, Dr. Janet Smith, I am on the board of timaron research. Dr. Daniel Peterson foundation he foundd and what we're trying to do is scientifically redefine CFS ME so we are strictly trying to do the science part, but today what I would like to do is plead with the FDA and with this panel to go along with the guidelines that just came out with the alZEheimers with approving drugs, I would like to plead that would go a long with looser rules for approving drugs for CFS ME, no approved drugs, thank you. [ Applause ] >> Thank you. >> Okay. We'll try one more time, Steven chillinski. No? Okay. So next we have Judy Mikovits. I know Judy is here. >> Dr. Judy, my travel expenses have been covered by several physicians and several patients. So I'm just going to read because I want to make it quick, I can talk forever. We do not know the causes of Multiple Sclerosis, parkinson disease, lupus or ME CFS. All of these serious debilitating diseases have abnormalities of the immune system and inflammation in common if not central components. We made a handout and many of you have it today, people who sponsored my travel. Of just a few of those biological abnormalities, clinical lab tests, and the drugs with potential for repurposing. The FDA has just approved (inaudible) immune modulator antioxidant for treatment of Multiple Sclerosis. The bright focus in drug discovery foundation just announced a phase I clinical trial of (inaudible) an FDA approved cancer drug which acts on RET noticed receptor like vitamin D and thyroid receptors, both abnormalities on the test. No abnormality tested in ME or CFS. In nor way, OVRNGologist just completed small clinical trial of FDA approved cancer drug rituxam, with success in 30% of patients. The logical next step to do in this trial and with the ampligen responders and nonresponders is gene expression and profiling and in fact immune profiling to determine the difference between responders and nonresponders at the molecular level. The FDA has demonstrated commitment by holding this unprecedented meeting and we thank you. Drugs which are FDA approved are generally safe in humans, of course everything has risks. In serious diseases, without treatments, classification FDA recently gave ME CFS the benefits far outway the risks. For advocacy groups here today and those listening I encourage you to fund these clinical trials with the drug companies, fund follow-up studies, profiling responders and nonresponders to divide patients that exhibit different levels of disease activity, prognostication and possibly insight into patho physiology as foundations have done. The technology and expertise exists. The absence of cognitive agent should not leave this field floundering any longer, it's a new era for ME CFS treatment. Thank you. [ Applause ] >> Thank you, Judy. And I think we got the documents but it would be good if you would submit those to the public docket so that they are officially included in the record as part of your presentation. Okay. Thank you. Next we have derek enlander. >> Good afternoon. I'm from the Mount Sinai Medical School in New York. About a year or so ago we actually received a million dollar grant from one of my patients and the dean of the medical school said, oh, that is very generous of you, let's actually form an ME CFS center. And indeed we formed an ME CFS have at Sinai, and our first project was to prove whether TET, was appropriate in the disease. We actually have got undergoing the research in post-exertional malaise and actually expect to look at 150 controls and 150 patients in this disease. We're actually going to look at the usual array of cytokines and immune markers, but we are also going to include genetic studies with a genet cyst Eric Shaft, who seems to be actually well known amongst all genetic people that I've spoken to. We've got actually a series of 39 pages of the JE nome study on our first patients. It is most remarkable genetic study and we are now actually going to look at whether in fact graded exercise therapy is correct method or approach and we will actually prove or disprove the pace idea. Thank you. >> Thank you, Dr. Enlander. If there is additional information related to a talk you want to submit to the docket, we would welcome you to do that. Thank you. Next Gisela morales, I'm not sure if I got that right, I tried to catch people whose name I wasn't sure of before. >> You are trying very well. Thank you for acknowledging that because not everybody takes the time to ask the question. First and foremost, thank you for putting this and make Thanksgiving happen. Compassionate way today, I'm pleased and happy to be here. I am Dr. Morales-Barreit, o, I am here today as caregiver (inaudible) love dearly and have been sick for the last six years. I'm not going to bother you with the symptoms, they are pretty much what has been said all afternoon. He has suffered a lot and we were blessed at some point in time to be -- to work and I heard it on MPR the voice of Dr. Peterson. So he moved a year ago to Incline Village and took him back a couple months ago, but he also came back herself again in many, many ways. Being active server of the symptoms that she has had has not been easy and watching a person that move literally at the speed of light be limited for a long time, to bed for days, months and years, really has been demoraleizing and emotionally draining. (Inaudible) survivor and when I had had cancer I had options for treatment. This population has really (inaudible) not really approved by anybody and guaranteed they are going to get cured. I'm here after almost 12 years and I know that if I get sick again God forbid, I can go back and find treatments that have been approved. For me, the big elephant in the room after everything that has been said is the fact that we need pharmaceutical companies, FDA cannot do it alone, and we need the pharmaceutical companies to come and play the game. The only way I think as psychologist is to also invite them with your approval, I think the FDA approval will open up the door for the pharmaceuticals to say yes, we can. Yes, we can help. So I saw the hand, but I really employ you to look into this from that point of view. If you approve any drug with whatever the name Ampligen will be nice, but if not, anything that will support the patients and open the door for more research and more pharmaceutical people coming into this. Thank you very much. [ Applause ] >> Thank you. And I skipped Mr. Thomas Equels. Close? Okay. >> My apologys. >> These don't work for up here and that one obviously not very good at either. >> Little tall for this one, too. First of all, thank everybody, FDA staff and most of all the patients and clinicians that have taken the time to come here today. I'm the executive vice chairman of hemisphere biopharma and we have an experimental drug that you have heard about, ampligen, I'd like to share a few words about how hemispheric and ampligen got involved with CFS. We were 30 years ago asked by the FDA to get with I believe Dr. Peterson and Dr. Lap with an unusual thing they had out there called the Tahoe Flu. And to provide drug on experimental basis for a female subject who had remarkable recovery. And I believe there were about about 12 additional subjects that were approved that did well. That was over 30 years ago and we're here today, and it's been a long and difficult journey for small company such as ours, but we're participating in this process because we know that there are thousands of Americans that are disabled. We know from the research that (inaudible) we believe we can contribute to that process. And we responded when you asked us 30 years ago to come to the table and work and we've toiled in this vineyard for many years and we make our commitment that we will be 110 percent with you and with you to make this happen. Now we know from the HIV Aids epidemic that when there are no drugs the results are dire and we believe that we're subsets can be identified where a drug can be effective, whether ampligen or other drugs, where there is unmet medical need there should be some form of expedited approval applied. I'd like to mention -- >> You have 30 seconds. >> Three articles that I think warrant a lot of study, causes of death among patients with Chronic Fatigue Syndrome, which deals with cardiac increases and shortening of mortality. Cardiac toxicity in Chronic Fatigue Syndrome, which deals with effect of you heard about 35, 40 different medications, all of which have severe, some of which have severe side effects and the impact and then a double blind placebo controlled randomized clinical trial of tlr-3 agonist, that study has to do with the exercise issues and how that may have an effect. Now we've expanded a COMBRAT deal of time and money to get to where we are today and I just want to say that we're prepared to enter into not a legal partnership, but a real partnership with the FDA, with the clinician and with the patients to bring relief for people who so desperately need it. Thank you. [ Applause ] >> Thank you. And thank you for paying attention to the hand signals. Mr. David Strayer. >> Okay. Okay. Dr. Dan Peterson. >> Thank you. I am here today representing myself as a caregiver but of approximately 9000 patients over the last 30 years which has given me -- this disease the frontlines, as they say O. Behalf of the patients, their families and even their physicians, I implore this esteemed gathering committee to not only device but to execute therapeutic strategy which is much needed. The Federal diagnostic criteria were established more than 25 years ago even though revised a few times. The CDC has identified counted, surveyd and queried an estimated 1 million people in this country suffering from the disorder. The attendance disability and poor prognosis documented worldwide by families, physicians and the patients. The estimated cost to our country 9 billion dollars a year. Diagnostic marker would yield the company 250 million a year. A therapy probably in the billions of dollars, yet 25 years later, we have no FDA approved drug for this indication or therapy. So the heterogenous nature of had disorder has been a problem and I see as an invitation or a beg to study subsets in this disease that have the same pathogenic mechanisms. Over this 25 year period of therapeutic stagnation, there have been thousands of pier reviewed articles published with respect to patho genesis, but it's been difficult to connect the dots, I'll just mention a few. Worldwide the number one immune logical marker is low MLK function and abnormal by everyone who look at them. Speck scans abnormal low stress testing is universal finding. Many clinicians are already utilizing markers and end points in their own practices as they treat patients to document efficacy or to teach us more about the disease. Symptomatic therapy unfortunately I think is useful in quality of life, but I have not seen it return patients to full functional physical nor cognitive conditions. I think targeted immune logical therapy has possibility to do that. On behalf of clinicians worldwide, you may not know this, we've already have established consortiums and collaborations worldwide with multi-primary care clinics that are ready and willing to do pilot projects, phase roam 1, II and III clinical trials and more and I know we're all committed to doing this. So I implore this committee to take some action to support the private sector, to use the resources and Federal government, science, personnel, computing capacity, to develop safe and effective therapies. Thank you. [ Applause ] >> Thank you. Mary silvey. >> Eileen Holderman. James baraniuk. I'm sorry, Eileen? Okay. >> Good afternoon, my name is Eileen, an independent advocate for ME. Thanks to the FDA for hosting this conference and giving me the opportunity to speak. I'd like to address a concern and that is the concern of the name of the conference which doesn't distinguish between myalgic encephalomyelitis and Chronic Fatigue Syndrome. There is one million American men, women and children suffering with ME, 17 million worldwide and unfortunately in 1988 the CDC renamed it Chronic Fatigue Syndrome, which is unscientific and dismissive and it was further compounded by definitions that included empire cal definition which just states one symptom fatigue to have it and today you heard multitudes of symptoms that really describe this disease. The results of the faulty definition have caused muddied research, the inability to replicate findings, no YOUN versal biomarker, drug development without target audience, erroneous medical information and websites, bad media and press, skewed public perception and most importantly neglect and harm inflicted on patients who truly have ME. The disease cost the nation billions in lost productivity, tax revenue and medical benefits. Funding for this disease has been low and it needs to be commiserate with the disease burden. The solution is that all the government agencies, the scientific, medical, academic, legal advocates and patient communities must come together and adopt the Canadian consensus criteria and to dismantle use of CFS and move research and treatment forward to help the over one million Americans with this disease. Thank you. [ Applause ] >> Thank you. Dr. Baraniuk. >> Thank you very much for letting me speak. I'm Jim from Georgetown. I wanted to applaud - crew for taking on this very great challenge, all the best. I also want to tell you that it is not all doom and gloom. We started publishing the results of our studies from Gulf War illness, patients who also meet Chronic Fatigue Syndrome criteria, only about half met Fibromyalgia criteria, we've identified three separate dimensions of exercise induced FRMI changes that we believe we may be able to apply to Chronic Fatigue Syndrome. First off, we've identified white matter abnormality that separates the GWICFS people from healthy controls. We have two neutrally exclusive, bold blood flow responses to exercise that subdivide into four neutral exclusive groups and I think begins to address the issue of heterogeneity, and these purely objective, you can't use any subjective criteria to define them in advance. The simple message is all of these subjective criteria that we're using for subjective syndromes we're going to get rid of. We're going to have objective diseases, we're going to have objective diseases that end up in Harrison textbook of medicine. Little bit since I have 30 seconds, my complex message here is that what we identified is a problem in the right interior frontal fACI culus, white matter that connects prefrontal lobe here that deals with fatigue and valuation of pain. It moves and connects with insula, which deals with phantom pain, the emotional link of how much pain means to you and it tracks posteriorly to involve the working memory, the default network which is your mind wandering or daydreaming that all of a sudden breaks up your thoughts, it also connects your dorsal and ventral attention networks, which are the systems for maintaining your focus. If you think about the cognitive dysfunction, the exercise induced exertion exhaustion, that is what our model is demonstrating abnormalities in and based on this, we're hoping we don't get sequestered and we actually get some funds to keep going. Thank you very much. >> Thank you. [ Applause ] >> And again just reminder to you and anyone else, anything you want to submit to supplement your public comment, please submit them in writing to the docket. Next we go to Mr. Charles Lapp. You have all proven me wrong, nobody has sat down, everybody came up to the podium. >> Thank you for this opportunity, I'm Charles Lapp, a physician from Charlotte, North Carolina. I've been treating patients with Chronic Fatigue Syndrome since 1985 and I've been using ampligen since 1988. My expenses today will be reimbursed by hemispheric, but I'm not here to speak for them, I'm here to speak for patients and say in the 28 years or 25 years that I've been using ampligen, we've had excellent success. I reviewed our records recently, and shows over 50% of our patients have responded very nicely to ampligen, and about 30% have significant improvements, which is sort of disconnect from the data. We chose the end point from the ampligen study many years ago and seem to be disconnect from the end point we are using to measure the effect compared to the patient global impression of change and the physicians global impression of change. We see patients who are treated with ampligen, who have seen remarkable improvement and return to gainful occupation, school and work. I guess the most important thick thing that I can say, two points I would make today, one is that in all of the years I've been administering ampligen, we have not had a serious side effect, not many drugs you can say that about. The second thing is that I think history will show that when a new drug is brought into a field, for example, when azt, was approved for Aids or interferron was approved for multimillionaire, many other players and FOORM suit cal houses that got in the field and opened up treatment of these two illnesses, we hope that perhaps ampligen will do that for Chronic Fatigue Syndrome. Thank you for your time. >> Thank you, Dr. Lapp. >> Steven lempert. >> I'm Dr. LECHLert, no financial interest. Approximately 70% of patients tested in the 1994 study of Chronic Fatigue Syndrome were positive by culture for hhv-6. There is subgroup of cfs patient with active hh6 for culture. Characterization by method chain reaction as indicated preNOM NANTly hhv -6 A, more frequently than hhv-6 b. In the paper by Dr. Blushy titled ampligen inhibits herpes, viral repcasion was inhibited by 46 to 98%. The inviTRO, antiviral effect reported for ampligen translates clinically to being effective inviVO in severely sick CFS patients. Mary was (inaudible) positive before ampligen and negative on amplegen three times. The drug converts hhv-6 infection into latent HERPes virus with marked recovery of both patient health and functioning. Patients severely afflicted with neurovigilent human HERPes virus 6 an occurs M subgroup with Chronic Fatigue Syndrome and progressive multiple sclerosis have (inaudible) effective antiviral such as ampligen, infusion is part of presumptive viral trigger in subgroup of patients with CFS. Antiviral amp, needs to be transferred to the FDA antiviral division and reevaluated now rather than wait another 10 to 20 years. Thank you. [ Applause ] >> Thank you. Mr. Dwight merriman. >> Joan, I know she's here. Sorry, grobSTEin. >> I'd like to sit down. Thank you. Hello, Dr. Joan, GROB, I'm a physician and had ME for 14 years, I think I might have said 13 earlier, I've lost count. FDA faced with great responsibility to encourage rapid development of treatment for this serious disease. There are several important issues to keep in mind in this process. It's important when evaluating drugs to make sure this patient population is well defined. I strongly suggest that the FDA require the use of Canadian consensus criteria for all studies. In order to better define patient population FDA should also make the validation of biomarkers very high priority. You have heard today patients have many measurable abnormalities. It's important to distinguish between therapeutic agents that treat symptoms versus agents that treat underlying cause of ME. Although the underlying cause is still unknown it's possible to treat symptoms and also possible to treat associated infections even if the infection is not the sole cause of the disease. It's very likely that multiple agents will have to be used to treat this multi system disease. This reality should be addressed in the design and evaluation of drug trials. Outcome measures must reflect the impact of ME on patients lives, small improvements in function are extremely important to patients and we're willing to tolerate risk to get improvements. Finally, the FDA should demonstrate a sense of great urgency to evaluate therapies for ME. As you know, there are currently no approved treatments, patients are paying for expensive efKASHS treatments like amp, and rituxin, out of pocket, causing financial harm on top of physical disability. It is FDA's responsibility to remedy the situation. Thank you. >> Thank you, Doctor. Jeanett e Burm. >> Okay. Nobody has paid for my expenses to be here. I'm here to urge the FDA to play a more proactive role in working with hemispheric toward approval of ampligen. Subgroup of ME patients identified as result of being amp responders and tremendous amount can be learn Friday that. One gets impression the aigence senot interested in the drug.It it is not even a topic at that drug development workshop. Dr. Peterson, the physician with the most experience and success in administering the drug and many other treatments, as well, has not even been invited to one of the panels. I'm happy to see he got two minute slot today. At the amp advisory committee, FDA conceded no path for amp not accelerated approval process. No explanation was given. The FDA recently developed new guidelines for accelerated approval process for drugs for patients who are not even sick yet. Why the drastic different standard I wonder? Looking back at approval of azt, as first drug to TREEFT hiv and aids it becomes clear the FDA does have discretion to adopt rule if the circumstance is certaintied. At the end of last year the FDA approved again under accelerated approval program a drug to treat tuberculosis five times more likely (inaudible) standard drug treatment for the disease without proof of inproved efficacy. I'm not so convinced the FDA hands are bound when it comes to approval of ampligen, it seems double standard compared to other diseases and other drugs (inaudible) cliff hemispheric is running out of money and the drug is going away potentially forever. Thank you. >> Thank you. And I have three more names that are not on the slide, but Mindy katei, I'm sorry, I don't know if I got that even close. Okay. >> My name is Mindy and I'm a science reporter and blogger at CFS central N. 1994, I wrote an investigative piece for Philadelphia Magazine called Aids Drug no one can have about the experimental drug ampligen, I'm here today to say it is vital that the FDA understands one thing, much of the data on this disease is useless because CDC and before that the NIH have not been studying patients with ME. They are not studying patients with natural killer cell defects, vo2 max abnormality or tilt table tests. They are not studying patients who neat Canadian consensus criteria for the disease or the international consensus criteria. [ Applause ] While the different definition can get incredibly confusing, you'll need to know one thing, the kuda, and revised kuda known as empire cal definition as well as oxford definition and the holmes definition of this disease are not accurate. Yet CDC, your disease definition instead of Canadian consensus and international consensus which are accurate, Dr. Leonard Jayson of dePaul University has shown in published studies that CDC in employing empire cal definition is studying patients with major depressive disorder, not patients with ME. That is like doing an hiv trial and none of the people are hiv positive. As a result of studying the wrong cohort doctors are misinformed. Dr. Lisa corbin said she tells her patients that "Monday is for mending, Tuesday is for ironing" I found her advice to be in a word clueless. But typical of the help any patients receive. Imagine give Thanksgiving Hokie advice to patients with hiv or MS, ME patients want and need treatment not patronizing proMIEDs. Five of the patients I interviewed in 1994 for the article have died, one a good friend of mine, three in their 40s and 50s. Do you really think that these patients would be alive today if only they had done their mending on Monday? Thank you. >> Thank you. Next is Mr. Don kalns. >> Hi, I'm an owner of small company called hiperion biotechnology. I am not really a CFS or ME person. We had audacious idea in 2004 that we could measure fatigue by evaluating changes in the composition of saliva. The army was very interested in this topic because a lot of folks fighting over there were profoundly fatigued. Since then we published a number of papers about the use of this biomarker, there is several small pep tides found in saliva that are quite informative. More recently, we have published or given a paper at the American association of clinical chemistry describing application of the technology to CFS patients. I have to caution that these were archival saliva samples, the Providence was not absolutely certain, but the poster presentation got an award at this annual meeting and pretty well attended meeting. I guess why I'm here ultimately is to pose a question. I need spit. I need spit from CFS patients, I need spit from control patients or not control people. There is a lot of flaws and I'll be the first to admit there is serious flaws with the SMOUL cohort we looked at in CFS patients. I don't want to step on toes or get any ruffled I'm very kog NIZent of the debockel, I would say that if well are investigators out there that have protocols that are ongoing, please talk to me. I'd want to get that saliva. I would require that the saliva sample sent to me are blinded, I don't want to have bias, if it doesn't pan out, it doesn't pan out. The big market for us is in assessment of fatigue in healthy people, not in sick people. But I really think this technology might have a lot of applications in terms of evaluating new drugs, in terms of efficacy, maybe in predicting crashes, there could be a lot of potential here. But come talk to me, I'll be here tomorrow, as well. [ Applause ] >> Thank you. And Mrs. Dianne lewis. Is she -- you want microphone Dianne? >> I hope I can do this, lie laptop hasn't been working all day. Dianne lewis, licensed certified social worker clinical therapist. I'm here to tell you as a professional this disease is not -- does not benefit well from CDT. But from a personal note, I want to say that the distance between life and death is but one step but the difference TWEEB living a daily death is life sentence with this chronic disease. The experience robs you of who you are, destroys your integrity, personality and stigma and discrimination that comes with this disease is uncalled for. For every attempt to step forward slow dying nature is no longer a step forward, but stepping backward with each passing day. Not having medical parity means we are nonexistent in life. I spend most of 75 to 80% of most days in bed at rest and it was easier for me as single parent working two and three jobs to earn what Two master's degree than to be sitting on the bedside. So today is a choice and I know I will suffer from this and I want to let you know that when I work I do four clinical hours of work because I stay within my boundaries. I really, that is really taxing me. In order to do that, I have to stay completely in bed rest, no contact at all possible on Sundays. I will work my four hours Monday, it will take me a long time to do my notes. Then when I come home, I am in bed Tuesday and Wednesday unless I have appointments and if that means I have appointments that extends that week. So I actually am living to be able to help people, but I am on palliative care plan, medications I get I've been told by our hospital administrator, we don't treat that disease here. You know, none of the doctors will consider this disease and so every single doctor I see or get referred to will only treat just symptoms that I would come in and say that was most disturbing. I invest a lot of my energy and time and efforts into energy conservation, knowing myself, trying to find out ways where I can actually achieve something, but know that if I have to use the cane, if anybody ever seen a social worker in a hospital we're on the fast roll, I have to have the cane slowing me down otherwise I'm going to burn out. I can eat a meal and my body will just drain of energy. >> You have 10 seconds left. >> But I do use a lot of hydrotherapy, benefit from soaks, other than that my medical interventions, you know, is mockery. I have been told to do so many things and the best treatment that I've gotten is because I have taken studies from Dr. Peterson and Dr. Lapp, and taken them to doctors and doctors will respect that research, then they will give me that medication I need otherwise I am waiting for the last stages. >> Thank you for your comments. [ Applause ] >> And for agreeing to be the last speaker unless I think -we covered everybody who didn't get on this list, but had signed up, is there any body who thought they were going to speak in the open public session and didn't get a chance to? We haven't gone over our time, so if there is anybody who didn't get an opportunity and has a comment that takes less than two minutes -- one minute, okay, that's it. >> I would like to thank the FDA for agreeing to this meeting. I would like to thank the advocates who helped to put this meeting together. What I'd like to kind of reaffirm and reconfirm is that FDA was willing in the days of hiv and aids to work with companies and bend the rules, change the rules, alter policy, try to figure out how to get a medication to people to save their lives. We now have I'm referring to as alz, plan which I would ask FDA to allow for ampligen. It's changing policy for patients who aren't patients yet. It is willing to give people who are not actually confirmed with Alzheimer's who knows what potent drugs that are coming out to treat these people. But FDA is willing to bend, it was mentioned that cituro was fast tracked December 31 of this past year, and that this drug kills five times more patients than placebo does, but somehow policy was altered because there is unmet medical need. There is unmet medical need right here and has been for a very long time. And so you know FDA is here and ampligen sponsor is here, there is a gap, we got to figure out how to bridge the gap. We need the right people in the room and figure out how to do that, how do we bridge it. FDA, the responsor, the expert clinicians you heard today and there are other expert clinicians who have given amp, who could sit down and help. Thank you. >> Thank you. Okay. I think that closes the open public session. You spoke during, we need to be fair here, this is I think time now that I turn it over to Dr. (Inaudible) to close the meeting and if there are comments that didn't get into open public session or if you gave your presentation and neglected to add something that you thought about later, please submit your comments in writing to the docket. And I really applaud the speakers for working with me and doing this without our timer. This session facilitated that, we can't always do that in open public meetings, but it was the right thing to do here and you proved it and worked with me. Thank you very much. [ Applause ] >> Okay. I just want to close by thanking you again for being here today. Thank you for sharing your experiences with what life is like living with ME and CFS. You know, terrible cognitive and physical impact you have been telling us about, the crashes you experience and the fear of crashing and the consequences of you're just trying to engage in daily life the way the disease has constricted your life and managing physical pain and other problems and challenges you have told us about it today. And thank you for sharing your experience with what you have been trying to do to treat the condition as best you can. And the range of therapies that you have described and things you have worked well and what has worked better than other things. I also want to thank you for being so generous with your time. And after spending a few hours with you today having a much better, I know I have a better appreciation of what sacrifice you have made and really how couragious you are to have come to the meeting today knowing full well as I now understand that you are going to have consequences for expanding the energy to be here and tell us how you are doing, so I think that is just a tremendous courageous contribution you are making to on behalf of this disease and other patients who couldn't be here, I want to thank you so much. And on behalf of this patient focused initiative, I thank you really made me feel you set us off to a very challenging start because we have to do our best to try to meet the level of contribution that you have made to us. This is as I said a first meeting and this is I think a first effort for us in this area to be trying to do better with trying to capture and describe quality of life and life impacts. As I said, we'll have to docket open to receive comments until August. We'll be producing a summary report capturing the information and having available a transcript and raw material so people -- am I doing that? Maybe. And we'll be posting that and sharing that with the reviewers and others and so this has been a very rich and challenging start for us. Thank you again and I hope that I know you'll have a very good day tomorrow if you are able to be there. I hope you have a good night. [ Applause ] >> Event is not active Day 2 test test. Test test. Test test. Test test. Please stand by. Test test. Test test. Please stand by. Test. Test. Stand by. Please stand by. Test. >> Moderator: Good morning, everyone. We're about ready to get started, if we could have our panel 1 speakers come up to the front. Yeah, come on up and have a seat. Good morning, everyone O. Behalf of the FDA, it's my pleasure to welcome you to day 2 of the drug development workshop for Chronic Fatigue Syndrome and myalgic encephalomyelitis. Day 1 was certainly an amazing day. I have to say I was so incredibly impressed by how articulate and topical all the comments were from the patient groups and also how much I learned, despite sitting through many hours of testimony, this was just really amazing I think in bringing out the symptoms and making everything so very clear. Thank you, thank you, to all of the patients who spoke yesterday, very incredibly helpful. We have an excellent panel lined up for day 1. I'll just do brief introductions. We have Dr. Bernard Munos who is going to be speaking on derisking drug discovery and he is the -- get the right title here, the founder of InnoThink for research and biomedical innovation. Following that we have a talk on drug repurposing, by Dr. Suzanne Vernon, who is the scientific director of the CFIDS association of America, and finally we have someone from the Office of Medical Policy at FDA, who is the associate director for Regulatory Affairs, Melissa Robb, who is going to tell us all about FDA's expedited programs for serious conditions and hopefully explain a little of the mystery behind some of the FDA rules and regulations that pertain to this area. Following that, we'll have an audience question and answer period and this is going to run a bit differently from yesterday. So you have all gotten these nifty little yellow sheets, if I can borrow one here. They look like this. These are for your questions, so please if you think of a question, just jot it down on one of these sheets. If you have more than one question, do more than one sheet and someone will be walking around, about 15 minutes prior to the end of the talk and will collect them and we'll bring them up to the moderator and we'll use that in order to get through as many of the questions as we can. It will also allow us to group the questions depending on on how many there are, it's possible we may not get to all of them, but we'll try to cover the ones that are most pertinent to the topic at hand. With no further adieu, we're going to go ahead -- I'm winging this a little bit, I'm obviously not Sandra Kweder, even though she has pretty big shoes to fill and I wear a small size, I think we'll get through it. So the objectives I think we covered day 1 objectives yesterday very nicely, engaging patients and you heard this yesterday, but today we hope to take what we learned yesterday and bring it forward to truly examine the common issues in drug development and hopefully consider some of the scientific and regulatory tools that we might bring to bear to move drug development forward in this area. Complex Chronic Fatigue Syndrome as you all know is a serious and debilitating disease, we don't know what causes it and we heard yesterday so beautifully articulated by the patients many of the symptoms and we are going to build on that today. As you know, there are no approved therapies and that is kind of why we're here. Just a couple of words about about the nomenclature, as we mentioned yesterday -- I don't know how that happened, Chronic Fatigue Syndrome and myalgic encephalomyelitis we recognize there is a lot of controversy about these terms, some people want to lump them together, some want to split them apart. At FDA, we've taken no particular stand on the name, we're use Thanksgiving to be inclusive, you don't have on have a name of a disease to have a drug to treat it, so that's sort of our approach to it. Again, we're using the term drug development to be very inclusive. And not looking just at what FDA can do, but what all stake holders in this can do to move the process forward. As you know, FDA has a very narrow focus M drug development, what we do, we do the regulatory side of things, so we don't run clinical trials on drugs, we don't tell drug developers what to develop, we look at the data hathey have developed and bring in to determine if a product is safe and effective to put on the market. So CDER's mission is to promote and protect the public health by assuring safe and effective drugs are available to all Americans and because of that, we have to apply careful judgment to scientific assessment and risk benefit balance and that is where patients come in. In helping us determine what that risk and benefit balance might best be applied to a particular disease. Marketed drugs have to be a blend of these, no drug is risk free, but what we need to do is make sure that we know what the risks are, that the riskss are managed, that quality in manufacturing and in other parts of the development process is assured, that we know what the trials showed, that advertising is appropriate and information is available to the patients on the drugs, both in the product label, in the patient handouts. We also have to ensure the drug is effective, studied with proper end points and standards and those standards have changed over the years. Drugs that were approved 25 years ago might not have the same approval now should they go on the market. So how do we do this? Well, FDA regulation began as interstate commerce, these were for companies who were intending to market drugs and usually these drugs development programs are funded by the pharmaceutical companies, they engage the academicians and community researchers to actually do the studies, but the pharma funds them and designs the studies. We at FDA oversee drug development assuring the safety and that appropriate regulations are being followed to protect patients and we work with their sponsors so the drug companies come to us with their programs and we provide them advice on these programs on how to best get their drugs approved. We also review all the findings when they are submitted to FDA. In order to do this review work, we incorporate a variety of different disciplines, these include chemistry, that's the manufacturing process, animal toxicology, which is required to even test a drug in humans, we employ statisticians and FDA statisticians actually take the raw data and rerun the analyses the pharmaceutical companies did in their NDA to show that the drug is effective. We employ physicians, such as myself, that look at both the protocols to ensure that safety seeming came, as well as review things from a clinical perspective to determine even approximate that statistical number is reached, what does that mean clinically to the patient. And we employ variety of inspectors both for the manufacturing plants and also for the clinical sites. We take all that and when a product is ready to be approved, we'll often take public advisory committee so that we can include the patient perspective. So what do we look at in between from when a drug initially goes into human with an investigational new drug application or IND, to when it gets on the market with a new drug application. We look at the chemical composition to make sure that the drug is manufactured in a safe way, we look at animal studies to ensure that we have some data knowing what the toxicities are if that is appropriate to give to humans. For example, if you had a drug that you gave to animals and every animal you gave it to died, that probably wouldn't be a good drug to test in humans. And then how you assure the safety, you have to look at the range of possible doses so that you can choose the appropriate dose and the plans for the clinical trials. You need to again look at doses, look at how big the trials should be, how you compare the drug, what the endpoints will be, you will hear a lot of these things as we go through our talks today and there are multiple points of interface between the FDA and the sponsors throughout this process, which can take a number of years. So the gold standard, this comes from the code of Federal regulations and this is what we consider to be an adequate and well controlled study as laid out in the law. It has to be designed well enough to be able to distinguish the effect of the drug from other influences, such as a spontaneous change, a placebo effect or bias observation. And an adequate and well controlled study, that is what we're talking about, the whole basis of the conference today is to talk about adequate and well controlled studys and what they might look like in this disease area. They prove valid comparison with the control, which is often a placebo, but could be an active control and they have a well defined patient population, so we'll talk about that a little bit. There are also adequate measures to minimize bias, such as randomization, you don't know which patient gets the drug, which patient gets the placebo. And methods of assessment of response are well defined and reliable. So this means that you have your statistical plan laid out, along with your protocol before you start the study. The analysis as a result is adequate and assesss the drug itself. In addition, you have to ensure that the conduct of the study is appropriate so people are record WHAG they are saying they are recording and it is (inaudible) fine, but it is not as easy in disease such as this where you're not quite clear what the end point should be, not clear what the patient population should be. So that's the struggle. And the clinical trial really matter. This is what makes or breaks the drug. It is essential to assess the safety and efficacy of a drug. The measures that you use for end points matter a lot because they have to be objective, easy to quantify or sign and those you have to know how to translate into how a patient feels, functions or survives, because that is the end game of where we're all shooting for. Subjective symptoms are fine, too, you just have to have a way to measure them and that is actually what we want, we want something that is going to make the patient feel better. Pain, fatigue, all of these are could be appropriate end points. And the patient also matters because the disease frames the whole scientific and regulatory considerations, the serious of the disease determine how much risk you would tolerate in a drug versus the benefit. But what's absolutely critically important is regardless of the disease, the standard of evidence is the same. So it doesn't matter if you are looking at orphan disease in a tiny little population or a disease like diabetes that affects everybody, or a disease such as rapidly progressive cancer that kills people in six months or a disease such as Chronic Fatigue Syndrome that is a serious disease, chronically debilitating, all of these diseases have the same level required. It's been mentioned some of the guidances that are out there, for example, for Alzheimer's , they do not change the standard of evidence that is required. So our agenda, I think we've gone over, and I know we're running a little behind today, so with no further adieu, I am going to turn the panel over to Dr. Kweder, and go ahead and get started. Thank y'all. [ Applause ] >> Sandra Kweder: First, I have to apologize for being late, I don't have any grand story to tell, I wasn't doing anything dramatic, I was sitting here making sure I got here for 9. I apologize, how many of you have ever been to a Jesuit school? Know about Jesuit schools, my son goes to school up the street.This morning I had to get up early he had something called Jug, Judgment under God, and it's replenishment, like your detention, okay, your Jug, he got Jugged this week and his punishment was he had to be at school at 6:30 yesterday in the morning, yesterday and today and tomorrow morning on Saturday. For some infraction which shall any unmentioned. He's a senior and I thought, crap, I'm going to get Jugged. But, yeah, really, I'll be back tomorrow. But again, I want to thank you for being here and I know that Dr. Michelle has already introduced who the panelists are, but before we do it, I think one thing that is important even though you know profession, about their professional background, I'd like to ask each panelist when you get up to give your presentation, if you would please just say a few words about how you came to do what you do now. Okay. Just a little bit, to get a sense of who you are, we kind of heard your educational a little bit, you talked about your educational background, did Terry go into this stuff? Even better, even better, I'd like them to each, I have their bios, okay, but that is all stuff. I want to know, I want you to tell these people where you come from and how you got to where you are in a snippit, if you are giving your presentation, they have a sense of what you bring to this topic and I think it is far more than what I could say that you printed out in advance for me. So, I'm going to get you, I will start with myself as moderator, I started my career at FDA in the antiviral drugs division. It was brand-new. When I came to the antiviral division in 1988, it was three months after the approval of AZT. Now unknown to many people at that time, there were multiple, multiple other drugs in the pipeline because ideology for HIV had been identified as a virus. So (inaudible) was not the only company working on this, there were multitude of companies working on this and the other associated viral infection that patients with Aids, which we called it at the time had. We couldn't measure viral load. Wasn't possible, we didn't have measures for it, we just knew it was a virus. Today viral load is everything and we spent antiviral division a number of years working with acedemicians and company experts trying to find a surrogate marker for Aids because the only end point you could study in the clinical trials was death. Okay. There was no marker, so today we don't look at that, we look at immune function, CD-4 cells. That was the second, that was the first surrogate. Today we look at that a little bit, we look at viral load, we can measure the virus. We approve drugs early based on on that surrogate marker and that's the accelerated approval, which you'll hear about from Melissa. But that was my introduction to the FDA. I've been at the agency a number of years since then, I have been in postmarketing safety side, I spent other years looking at other effective drugs and the past 10 years I've been where I am today, which is in the Office of New Drugs and my Office on the Deputy Director and we oversee the clinical therapeutic division for all drugs and therapeutic biologics. So I am going to turn this over and we are going to begin with Dr. Munos. Why don't you come on on up and tell us a little bit about yourself and get started on your talk. >> And Mary Gross, you can come take my lunch money. >> Good morning, everybody, thanks to the organizer for inviting me to make this presentation today. As you can tell, I have an accent, I am not born and bred here, even though I spent most of my life in Indianapolis, I came from Europe, in France I got my first degree in animal science and came to this country on scholarship to study first economics at University of California and business at Stanford, joined industry in 1980, and have seen it fall from grace over the last 30 years when I was active, (inaudible) 30 years in Indianapolis. Basically because I saw it going from where we call culture innovation, which made working in the industry in the '80s absolutely exhilarating, one of the best times in my life, I couldn't wait to go to work in the morning, it was that good.That culture changed in the '90s from what had been culture of creativity and IB innovation to a culture of process. Initially I felt uncomfortable about this, but I didn't know why and it took me about 10 years to figure out it was impact that this change had on innovation that was worrying me. This was an industry spend tens of billions of dollars on research every year and yet no one had a clue about where (inaudible) comes from and how to get more of it and I thought that was mismanagement on a grand scale and no one was even worry body it. I said, somebody needs to do something about it and I foolishly perhaps decided I would be that person, I would start it. And did some research, publish someday papers, got some attention and the more it went, the better we started to understand what actually produces (inaudible), so today, 10 years later, we're miles ahead of where we were just 10 years ago and I work with some of the findings in my presentation. >> Wasn't that more interesting than what I would have said? >> Bernard Munos: The problem that gathers us here today, how do we get more innovation in CFS and why don't we have innovation there in the first place like M HIV, or number of other fields? Well, the first thing that you discussed today that we know is that CFS, the problem innovation in CFS compounded by the fact this is a complex disease that is often mistaken for other diseases, its borders are fuzzy, we have ideologies, it is not a simple thing to deal with. And the traditional drug R&D model is not (inaudible) to tackle something like CFS. Just for those who are not familiar with the pharmaceutical industry, I will take two seconds and describe how it works. We have scientists in the industry, drug hunters, they are very talented people who basically look at what is happening at the frontier of science and when they see something that is really interesting, really breaks through the distinct they can turn into commercial product that patients can vie, they will start a program of translation, which is basically a whole series of scientific activities aimed at churning that scientific discovery into a commercial product, physical product and get it approved by FDA. So that is the first problem is there, I mean CFS not being an established discipline on its own is sort of like cancer or like diabetes where scientists can really follow what is happening in the science in those areas. There is not science of CFS or there is, it is not well established one, so they may see things that may relate to CFS, but do not relate them as such, so there's adverse translatable research, surge of (inaudible) scientists can readily identify that could be translateed into novel drugs of CFS. We have consequence of that also, lack of infrastructure, basically defined as data and tools, even though we've been working in that field, you have been involved in that field for many, many years, it's not where it needs to be. I talked about the fuzzy borders of the disease and the fact that many symptom are often misdiagnosed for other thing, that doesn't help tjust introduces another dimension of confusion. The treatment address the symptoms, not the disease, if you go to patients like me for example and look at the CFS community, you will find all sorts of things, literally dozens of drugs are being used, all of them to treat the symptoms, not the underlying cause of the disease. Let's face it, if you are dealing with such a situation where things are so confused and ill defined it becomes very difficult for regulators to really regulate the science in that particular area, you know why is the natural history of the disease, what is the cause of the disease, what are the end points, unless you have that information or the information to analyze the question, it becomes very difficult for regulators to do their job. So the job remains in front of us, we have to do it. I would propose in the rest of my talk that what we need in CFS is what I call the development of an innovation supply chain. It's not all that complicated really. We need what you might expect intuitively, well, the goal is if we want scientist to work in CFS, we have to make it worth their while, to allow them to basically make it easier, cheaper and faster to work on CFS. Scientists tend to follow the path of least resistance, it is smart of them, and so if you have the infrastructure, tools, the data that make its easier and cheaper and faster to work on the disease, that is where they work from, they follow the data for reason that we'll talk about in a few minutes. So we need data, we already have some, but not enough and not in right format and not good enough. We need more and better data. We need tools to go along with the data. We need partners, we need money and lastly, we need leadership and passion, I should stress already we have quite a bit of that already, but need to connect it a little bit better than it is connected today. So we need data, that seems to be obvious, there cannot be any science without data, but it doesn't hurt to remind everybody and there is another quote there that I think is worth bringing to the attention of this group. If you think about the scientific revolution in history, they have been driven by one thing, the availability of data from (inaudible) to quantum mechanics, data drives innovation. So let's keep that in mind and in case of CFS, the data challenge is magnified by the fact it is as we know a disease that is fairly heterogenous and therefore to understand what is going on there, we need more data than we would if the disease were simpler. We need lots of data, we need patient data, we've got already patients registries in this country given the complexity of the disease, it would be even better if patient registries were (inaudible) lead us to better understanding of the disease. The natural history of the disease is something that we know to a certain extent, but we need to get better and thankfully the technology allows us to do that. We've had discussion already with the IDS foundation and you have got technology today that allows CFS patient to track mobility, track efforts with data, collected effortly by bio sensors, cell phone, it could be this thing tracks how many stairs you climb per day and how many miles and when you actually do the activities, not only can you track the expense of the physical exercise, but also intensity and all that can be collected effortlessly and get thousands of patients who are doing that at the same time, pretty soon you will have serious data for scientists to work on and all that hardly costs a thing. So I think it is one thing for the patient community to think about it, band together and avail themselves, technology in order to provide scientist with this natural way to start, natural history of the data and it is even more powerful if the natural history can be connected with genomic data. Up until now really, that was a dream, that dream is becoming true, it takes right now 5000 dollars to genotype someone and that cost is expected basically drop down by 65% every year, so that cost is expected to reach a thousand dollars before we know it and at that time it may be worth considering at least offering the CFS patient the systematic free genotyping of the genome, so scientists have more data to work with. That is all the data collection efforts is and likely to be done by anyone but CFS patient. The industry will not do it. Because again, the disease is ill defined, we don't have special list of CFS. So the patient community really has to take that in its hands, but as I said, it is becoming possible to do that on a short string and therefore should be considered. Technology as I put it here makes it possible to collect high quality data cheaply. Data that FDA will love because it is collected in real time, it's sent wirelessly to computers, there is no possibility for intervention, for editing the data, it is high quality data. And so that is the first point, we need data, better data and it is in the hands of the patients. The good news is if you built it, they will come and we know that because I mean the scientists will come, we know that because they do every time. (Inaudible) multiple myeloma, at the time not a single drug approved for the disease and she decided to do something about it. What she accomplished it tells you, gives you idea what is possible. Since 1998, she raised $225 million, sequenced the myeloma genome, started 45 trials, 23 drugs, six approved which are provided to the patients affected. And interestingly enough, the incidents of myeloma, in the population, the size of the patient community is smaller than the size of the CFS patient community 4 per 100,000 and 7 to 3000 for 100,000, judge the breadth of the bracket tells you how fuzzy the border of the disease is. So if you build it, they will come. So it is worth doing. We need not only data, but tools because let's face it, can't be much research without tool and data without tool doesn't get you far, tools leverage the value of data. (Inaudible) nothing new there, they are what scientists need in order to do their job. Tissue banks, talking about animal models, biomarkers assays and interestingly enough today you need networking tools so the scientific working on CFS can better connect with each other, this is as we have found from research an essential ingredient in innovation, ability for scientists to cross pollenate, not just with the scientists that surround them, but people half a world away that work in the same field and have completely different ideas (inaudible) -- successful, so if one kid, college kid can do it, the CFS patient community can do that for itself. And most data and tools in order to be helpful need to be in open free access to everybody who wants to work on the field. I know you get the pushback there, the data and so forth, data is not competitive, data is pretty competitive and should be shared. Everything upstream is noncompetitive and should be shared, that's -- we should collaborate in the science and better compete on the drugs that incorporate the findings on the science. The good news, if you create the tools, the scientists will come and we know that because they do every time. The example there is initiative called open source drug discovery platform or project started in India of all places in 2008, when the Indians realized that tuberculosis was a big problem there and that we haven't -- don't have a lot of cutting edge science in the field or effective drug for that matter. (Inaudible) computer platform developing new treatment for tuberculosis. Today that effort generates two-thirds of the paper published in the field of tuberculosis and the whole effort runs on less than 2 million dollars a year. So I mean it is hard to do cutting-edge research cheaper than that, it can be done in India, it can be done here. We need partners (inaudible) young investigators (inaudible) knowledge of the disease and there has been very interesting research at MIT, done to suggest that actually scientists or physician, I'm sorry, physician working with patients for whom no treatments are available have been historically very powerful source of innovation, possibly the most powerful source of innovation (audio cutting out) -- This is what you need to get the industry interested. From that point on, I think you can start looking for partners, corporate partners, big companies, but also small companies that can take the tools, the that you have got, the data, the discovery that you have made and start helping you out in coming up with new drugs. The regulators, they are keen to help and you can help them a great deal, especially in the area of risk assessment and trade off. Every drug approval process involves risk assessment and trade offs and the patients are the best people to make the assessments and trade offs. The patients are the people have regulators design trial protocol, for example wHIV, they know what risk they are willing to take and they are the only qualified ones in my view to speak about this. The scientists, companies, regulators, if you ask them, they will respond, they will respond because as far as scientists are concerned, for example, good rule of thumb is 80% of the time and the cost involved in research project is cost of generating and curating the data, which is work necessary, but frankly not very rewarding. If you offer the scientists (inaudible) basically multiply their productivity by five fold. So the scientists will come, because you make it easier for them to do high quality work then there are other options. We also know that passion shortens time lines, lowers cost and raises the problem of success. It sounds perhaps unexpected, but if you want proof of that, look at the foundation that are being created by parents who have a child that may die and they are determined to save that child, you know nothing about the pharmaceutical industry, scientists, don't have money, but will save that child and create foundation and (inaudible) specialist of the disease and talk to the most specialist in the world they can find and those specialists oftentimes find it irresistible to or hard to resist, hard to say no to the parents that are driven by level of passion and before you know it, those parents who started with nothing have research program, they have scientific advisory board, tools and data out there, compounds in development and drugs on their way to the market.It happens all the time, it happens with diseases that are much smaller than CFS. So it can happen here, too. And lastly, we all know that it is more exciting to work with people who are passionate and for whom failure is not an option, just keeps life more interesting, they will respond. Money. Yes, we need money, but the good news is it getting cheaper and in fact getting a lot cheaper. We have all kinds of new research model. You probably all have heard about the billions of dollars it takes to come up with the drug, not because -- it is very hard, but also I think this is an industry and fortunately where waste is there is model that make it possible to run very audacious research program on shoe string, you may have heard about the open source model, the crowd sourcing, virtual pharma, we don't have time to get into public-private partnership, drug purposing and better variety of model to choose from and each one of them has potential to deliver new drugs. The CFS community is larger than communities that have been successful and already it can be done. You need leadership and passion and it is already there. The CFIDS foundation was created in 1987, so it's got 25 years under its belt, during which it has raised over 30 million, not a trivial amount of money and that effort can be continued and expanded, especially with support of the patients. There is network and infrastructure required, not starting from 0 like parents who find they have a problematic child, well on your way and it can be done. Good luck and thank you. [ Applause ] >> That was a perfect start. We're going to do most of our discussion after the three speakers, but let me just ask if anyone in the audience has any clarifying questions, not a comment or long -- clarifying questions, anything that Bernard said that you didn't quite want to clarify. Yes? Right here, over here. >> (Inaudible) -- >> I tend to use industry jargon, patents and secrets and all that stuff. >> Secret stuff. Okay, Dr. Michelle, did you have a question? >> Just to clarify the point that (inaudible) jot questions down on the sheet and we'll bring them up and -- >> Yes, yes, you should have the yellow paper. Okay, thank you, Bernard. Good morning, everyone. My name is Suzanne Vernon, the scientific director of the CFIDS association of America and I got there by way of the Federal government. I actually started (inaudible) (audio cutting out on telephone) -- many ways changed the landscape for women with cervical cancer. That for me solidified the idea of really wanting to work at the CDC, where you could put powerful technology to play in populations and really tackle public health problems like no other place could. And we did so in 1998, we had the opportunity to start a laboratory program to really identify some of the underlying biological underpinnings of CFS. And we worked together with a large team of people for about 10 years and that program is still going on on, I think the thing that was interesting and important to me was again this idea of being able to study large populations with powerful tools and generate a lot of a lot of data. And the problem was back 10 years ago, we didn't have the kind of techniques, technology to really wrap our minds around this data and so over the course of a couple years we started putting data out there and engaging people with ways to really try to interrogate information, analyze the data and pull things out of it that maybe we wouldn't see with just the naked eye. And in 2007, the opportunity to really bring some of this I think incredible approach to bridging basic research, basic geneology, to having impact in patients happened with the opportunity to work with the CFIDS association as a translational research leader in CFS and I jumped at that opportunity because I think we're AT&T a very important time, then and now, even more so now where we can really take this incredible knowledge that we have accumulated over the years in the basic research arena and use that to really make remarkable advances in CFS. So that's how I got here, that is my road. Very interesting one. So I'm going to talk to you today about knowledge and intuition for drugs for CFS. The basic research, the epi, and the bedside helping to provide infrastructure for basic researchers to bring their discoveries to impact patient communities. This is really what is known as derisking science, making it attractive to industry in order for them to pick up the ball and again impact our patient community. This has been our innovation pathway over the past five years to really provide an infrastructure which allows that information flow, those discoveries that occur at the bedside to come across the way and be attractive investments for pharma. One piece of our infrastructures are (inaudible) biobank, that includes sample repository and registry. To date we have 800 ME CFS and healthy control. We are a partner with pharma, and we have a number of funded investigators both funded investigators and investigators interested in use Thanksgiving resource already using it. And this has happen immediate three short years. So it's a remarkable demonstration that this was a really truly needed aspect of CFS research to really get people interested as Bernard said, if you build it, in fact they do come, but you have to put it out there and make sure people understand what the resource is. We've also built a knowledge base and data analysis and data sharing platform, which you will be hearing more about in the coming months and this will be open and available so it will provide a tool source that is needed in our community to help with the aggregation and analysis and sharing of data that we generate. And as Bernard mentions, the CFIDS Association has been around since 1987. We are an incredible resource of knowledge with chronicals, with every publication that has been printed over the years and this is also going to be made publicly available online at the push of a button, indexed and everything, very soon. And importantly, we've really sought out new research avenues with drug not necessarily new it's become attractive again because of the technology that we now have available to us as far as knowledge and databases to query. This is just a summary of (inaudible), we know we call it a number of different things. To date, there is 6000 publication in Pub Med describing every aspect of CFS, from the basic path of physiology shown in the picture to the epidemiology which describes one million people in the U.S. with CFS or 17 million worldwide. We've identified many of the CFS risk factors, we do understand the patho physiology, we do understand tremendous economic impact of CFS and we now know as evidence by this gathering FDA considers CFS serious condition. Still there is some limitation, over the past 25 years there has been about 150 million dollars spent by the Federal agencies on on CFS to provide, gather all that data shown above. Still causes, a cause or causes have been elusive, we still lack validated replicated markers for diagnosis and treatment. For that, we haven't figured out or do not have a regulatory framework for CFS yet and because of that, we still don't have an FDA approved treatment and we have no standardized widely accepted clinical guidelines and because of that we still have our physicians, our wonderful physicians grappling with CFS and managing their patient population really with symptom-based treatments. Still the good news is we have a foundation of which we can build on on, we have incredible knowledge base and knowledge expertise in this area, which we can build on. This is why (Inaudible) suited for CFS. Drug repurposing is great for diseases that have clear unmet medical need, that are multi factorial in disease patho physiology, because as you see in this picture (inaudible) -- Drugs that have known mechanisms of action that work in the specific area or patho physiology, but it can also be used to identify biomarkers, the work that I'm going to talk to you today is drug discovery work. (Inaudible) number of symptoms and markers in CFS. Next highly correlated with delta (inaudible) and HIFTa mine. Seratonin, there was this really strong association with monotransmitters with very many aspects of CFS. (Inaudible) blue line is Chronic Fatigue Syndrome, on the X axis, are a number of different drugs in the database and this is the e-health adverse event database. On each Y axis is the frequency of the adverse event. This Y axis is frequency of Chronic Fatigue Syndrome, that Y axis is frequency of exhaustion. What they found was that the frequency of exhaustion and chronic (inaudible) medications being used in this population may be contributing to the system constellation. This was further validated in another database and this is the (inaudible) FDA's adverse event database. On the right, you see e-health drug that are all associated with increased levels of exhaustion or Chronic Fatigue Syndrome. On on the right is the error database and lines show that the same report was found in both databases. The drug in red were also drugs that were reported to have an increased level of fatigue or low efficacy from our bio bank. Three different data sets validated the same findings. (Inaudible) counter TUative finding that this particular mechanism could in fact be associated with fatigue, but now it is actually sitting quite nicely with mechanism that is being extensively described in the literature called central fatigue hypothesis. Drug repositioning identified two drugs and that I didn't show you, but two of the drugs can be used in combination to address two to three CFS symptoms. Validated by adverse event databases and by data in our biobank and currently in the process of designing a proof of concept clinical trial to test these drugs as combination therapy. A very exciting result from a partnership that literally started one year ago. We need to tap into our physician knowledge base and we designed a tool to capture clinical intuition and this is based on a meeting that we had at cold spring harbor last year in October and Beth Unger, said we need to figure out how to capture clinical intuition. We partnered with Bio Vista to develop a web-based tool and this is shown here. They would review it, mark it, they would indicate whether or not the drug had the efficacy level of the drug and then we basically analyze the results. I want to read this quote up at the top from Dr. Kweder. The key is that a lot of the research in this to date has been out on their own, researchers, clinicians are following series of (inaudible) no one has been able to tap into the information they have. This was a clear effort attempt to do just that response was great and very valuable. Another complicated graph on the left, on the Y axis, is the efficacy level from not effective on the bottom to very effective on the top. On the X axis, are symptoms they were queried about and then each dot, represents represents a drug they indicated had some level of efficacy, the only ones I'm showing are ones that had somewhat to very effective levels of efficacy. (Inaudible) some ability to impact symptom CFS at the symptom level management from very effective level that this information was also (inaudible) bunch of different knowledge to make connections that would otherwise have gone undetected. (Inaudible) age can clearly delineate CFS subtypes. (Inaudible) we provide these in open ended text format, so not to bias any of the responses, we divide these up into disease impact, symptom and treatment and we analyzed it using some technology and pretty sophisticated part of speech parsing using natural language processing algorithm, and the important thing is we tried to standardize coloc WIal response by matching it with unified medical language system. Now this is the answering. There is 500 million words. No, is that right? Half a million. 50,000, that's it. 500,000 words and 1200 responses and those are those half a million words up there. Now, this is shows you that there is a lot of challenges to dealing with colloquial responses, but look on the diganal, you begin to see very obvious clusters. Despite the fact there is a lot of noise in the system. There is a few interesting clusters that are popping out. We're in the process of now zooming in and each cluster contains about a thousand people and their corresponding bits of information about treatment or about symptom or about impact. So that is the diagonal, right there. Let me share with you a little bit of the very general high level responses, so from symptom perspective, we came up with three predominant clusters of symptom responses from patients. One being described as muscle pain, exhaustion, sickness, joint pain, malaise, fatigue, sore throat, brain fog. Another was very much a pain subtype described by pain in all parts of the body, very distinct group from the rest of the group. And then another one was described as exhaustion, malaise, weakness, spasms, sensitivity and sore throat. The message from this is that clearly the patient population is providing information, has information that can allow us to begin to subtype, identify phenotypes we might not have found otherwise. There was not as much information from treatment realm. Thank you. The biggest group of responses in the treatment domain of the survey responses said that there was a big use of nondrug therapy, nutritional therapy, diet modification and antiinflammatory drugs. The next was a pain, using PREDnisone, which really was used to manage again mostly pain symptoms and then the third group was really an activity modification group, so using a lot of pacing to really manage the disease well. Our next step in drug repurposing is again to get underway with submitting our pre-IND, documentation, for the proof of concept clinical trial and to expand the (inaudible) biobank because that will be a main part of the participation in the trial and other trials work optimize clinical information platform so that clinical intuition platform so we can get more input from physicians and that will help us really understand patient phenotype and comorbid conditions, which are very common in this population and then operationalize patient centered path and active data collection using some of the devices that Bernard had mentioned, as well as providing data sharing platforms that are user friendly and engaging and sticky. So thank you for your attention, I'd like to thank bio Vista and thank the physicians and providers who provided the clinical intuition data and I would like to show my thanks and appreciation to the patient ME patient population, family and friends for really jumping in with us and giving us data to make this happen. [ Applause ] >> Thank you so much, Suzanne Vernon. Our next speaker is Melissa Robb and I want to state the obvious, that we are a little over on time. We will allow this session to go over, we will still take a 15-minute break, but we may end up shortening the lunch by a few minutes to make up that time. I think the basic topics are really, really important and I want to make sure that we lay firm foundation for the discussion and the rest of the day. >> I've been with FDA for little over 10 years, started in a review division as project manager. About five years ago in 2007, when the food and drug administration amendment act was enacted, I started working closely in developing different types of programs that spanned lots of drug classes and was working mostly on post-marketing initiatives new to the agency by new authorities given by Congress with that legislation in 2007. In 2012, the food and drug administration safety and innovation act was passd and there was a lot of new authorities around innovative and expedited programs for drug development focusing on serious conditions and also on areas of unmet medical need. So that's really what I'm talking about here today is the focus of the agency is putting on innovating and bringing new products to market and bringing them to market quickly. So -- I don't have any disclosures, I work for FDA. I'm going to give you a little background to set the stage. I mentioned (inaudible) the safety and innovation act of 2012 and thinking back from that act, that is not something new for FDA to want to faSILGtate and expedite drug development, it is something we've been doing for quite a long time and have a lot of regulations in that area. Specifically I think the first time we articulated our thinking on speeding availability of new drug was sub-part E regulation we put out in '88. And what that did, really talk about the idea or general philosophy that FDA had toward assisting development of new therapies for serious conditions especially where there exists unmet need. I think you heard from Sandy and some of the other speakers that while we want to facilitate and expedite drug development that doesn't alter the requirement for safety and effectiveness and we're not lowering the standard, we're looking at other ways to provide the needed evidence so we can be assured that drugs that are approved are both safe and effective and have appropriate risk benefit assessment for the diseases that they are intended to treat. So following the sub-part E regulation, the accelerated approval regul AGSZ will put out in 1992. I'll talk about these quick overview time line thing. Also in 1997 the fast track provision were put in place and also -- I'll get into those in more detail. So like I mentioned (inaudible) part new law in 2012, title 9 of that law specifically addressed expedited drug development and reenforced the commitment of FDA to expedite drug development clarified some of the approval or requirement or accelerated approval which had been in place through our FDA regulation and had been initially put in place through earlier congressional laws. It created a new type of program called break through therapy, which I'll discuss a bit more and required FDA to issue draft guidance. On accelerated approval by July of this year and on break through we had 18 months so that is not until January of 2014 is due date. So there are four existing programs now. Fast track designation, break through therapy, accelerated approval and priority review. I will talk about each of them. Before we talk about them, I want to get some ideas or set the stage for common terms that are present in all of those programs. So serious conditions is (inaudible) is obvious unmet medical need if there is no approved therapies. However, some conditions even with approved therapies can have an unmet medical need and be eligible for some programs. So I'm going to start off talking about fast track and the first way that I organized them, they kind of are organized by how these programs would be used by a sponsor to facilitate drug development, so this is program intended to expedite development of molecule for FDA review and the clinical development time. Fast track is intended for serious conditions that have unmet need and you would be eligible to have get a fast track designation if you had data demonstrate potential to address this need. Now the data for fast track can be clinical or nonclinical and that is an important distinction when I talk about the program break through, but in essence fast track is much broader the eligibility, you don't have to have so much. But what FDA tries to do is expedite the development, meet with sponsors and discuss design in ways to make the drug development program more efficient. This is a program that we've had for almost 20 years now, it is not something that was new that we've been doing. It does have a component that affects the review time and that it allows sponsors to submit complete portions of an application through what is called rolling reviews. Typically sponsors have to submit, get all data and analyze it and have complete package before they submit new drug application. Biological license application and NDAs or BLAs. But it is sponsor has received fast track designation, they are eligible to submit parts of their application as become available. The goal well really is to expedite the review process and get the drug to market quicker. Break through therapy builds off fast track, another program intended for the development part of the clinical development part of and not the review phase where it is at FDA, but where the sponsor trying to develop the evidence to submit and the criteria here is that it is a serious condition and that there is preliminary clinical evidence that indicates the drug may demonstrate a substantial improvement over existing therapies. And so this is intensionally higher bar than fast track. But where it gets you is there is commitment with CDER and Cerebral Palsy implementing the provision to work closely with senior managers and experienced review staff to look at opportunities to shorten and the development time line. There is cross disciplinary review and looking at impacts affect approval outside of the clinical development looking at issues like manufacturing and inspection and different ways to make sure the whole package is ready to be to market as soon as possible. So break throughs, we don't have tons of experience with it, we have designated a handful of products already.It came with the 2012 legislation and so it is already being adopted and I think we see it being used in rare disease community. But also in other types of indications. So accelerated approval is a little different than break through and fast track. Break through and fast track are useful tools for sponsors or products trying to get facilitate drug development. When we're thinking about accelerated approval, we're thinking about how the end point we're using to approve a drug. So we still have that same requirement for safety and effectiveness, but we're using different end points to establish that. So the regulations, there is regulations in Federal regulations for drugs and part 601 for biologics. Some important clarifications that they put in and that was legislation from last year that allowed additional flexibility and clarity to how the FDA implemented theac cellerated approval program.It allowed the FDA to take into account the availability or lack of alternative treatment in making a decision if there were drug development programs that should is be considered for accelerated approval.It clarified the type of end point that can be used. Traditionally, FDA used often surrogate end points for the use of approvals accelerated approval. (Inaudible) talks about the use of clinical end point which we've adopted this term intermediate clinical end point that can be measured earlier than irreversal morbidity or mortality, but likely to predict that or another clinical benefit. So the key to accelerated approval is use of end points that are different that we see with traditional approvals. Accelerated approval has many criteria similar to other program, only for serious conditions, it's for you need to show or establish MU NIS Pap therapeutic benefit over available therapies and you also have to show that it is reasonably likely that the end point that you are using will predict ultimate benefit you are seeking to impact in that disease state. You have to show two things with accelerated approval, that the end point you have shown is reasonably likely to predict the benefit and that the safety and efficacy with that end point. So the big advantage is it can shorten the time to approval, often surrogate end point are clinical don't take as long to measure, so when you are looking at measuring a chronic disease state, you can find a measure that predicts your ultimate goal at earlier time that can save a lot of time. Chronic disease and where you would have a drug for extended period of time to get the ultimate outcome you are interested in knowing about. And the surrogate clinical end point may occur rapidly or sooner and then that informs the approval. It is has been used extensively M cancer and HIV, especially Sandy eluded to use of viral load, which is very common in the early days of HIV.It does have certain requirements associated with the approval. One is the research nonpromotional material they have to be reviewed by FDA prior to being DIZ sem NATed, but also required post-marketing confirm trials to show that the evidence that you prove that is reasonably likely to predict the benefit is (inaudible) trials. The this is a program that is intended to shorten review time. The fast track and break through will help developers the goal to shorten development time (inaudible) with prescription drug user fee act in 1992. And what that did is acknowledge that there was a gap in FDA review of certain of all applications and try to find additional funding and resources to support certain expectation of the FDA. So with that came priority and standard review. (Inaudible) demonstrate potential to be significant improvement in either safety or effectiveness and then the goal would be you get shorter review time. All the programs especially the development program is the one that have passed development stage focus on importance of early communication between the sponsor and the FDA. The goal is really to facilitate drug development to help look at study designs that optimize the way patients are enrolled, optimize the collection of data in order to make studies and drug development more efficient and have smaller, shorter trials. And emphasis on the new regulatory science and evolving understanding of the mechanism of the diseases are very important in order to implement these approaches. So looking forward, we're currently working toward getting the draft guidance that is required by the legislation out by July of this year, there will likely be comment period, there will be asking for any thoughts on the guidance and our intention is to be putting out one guidance that discusss the programs in order to make easy to understand where there are most appropriate and how to be useful and then following that will be issuing final guidance also. And on this slide, a couple resources that might be useful, currently some information sheets around and fast track to reference on the FDA website and a fact sheet on break through and 2012 drug approval report that CDER put out discusss some of the different types of development programs that were approved during 2012 that took advantage of some programs. I guess it is important to note thaof the different types of development programs approved through 2012 that -I guess it's important to note that these programs aren't mutually exclusive. We do see many drug development programs that take advantage of more than one of these. And that's great. That's what they're intended to do. They all have different contributions they can make to drug development and getting patients access to these important life changing medication sooner. That's my talk. >> That's great. Thank you very much. Before you sit down could you just stand up there Melissa where you're standing? I want to point out that she's wearing a uniform. She wears this every day. I don't wear this one every day, if you look around the room you'll see several people in uniforms. You can sit down. There are other folks here and a couple people asked me about it yesterday so I encourage you to ask them about what they do at FDA but we're all commissioned officers in the U.S. public health service. That's not the navy, not airline, but if you think about the surgeon general, that's of the U.S. public health service. We do not have guns. That would be a real mistake to give healthcare providers weapons. And but the public health services about 8,000 officers today. We're stations in places like FDA NIH, we have people all over the world. And with do the same jobs that our non-commissioned colleagues to in the agency but in addition we have other requirements readiness requirements, understanding disaster relief and we'll called button in times of disaster to deploy. Last week a team of mental health professionals from the commission core were sent to Boston. Team of mental health professionals were sent to Newtown Connecticut in December. We basically ran the recovery -immediate disaster activities at hurricane Katrina. That's who we are, so you can examine their uniforms. We have a number of questions here and I want to thank the panelists this is one of the most interesting sets of presentations that I've had the pleasure of listening to and particularly moderating. We have a couple of questions about where can we find the material presented by the panelists. I believe the slides are going to be available on the FDA website where you'll be able to link to this meeting. So you can see them up close. I had a small version of the slides. I have a number of questions here about you want to ask the panelists if you have questions for each other or comments for each other. >> I have a question for Bernard. I lie you alluded in your presentation to the fact that CFS appears to be on the right track as far as being able to to be innovative in a disruptive way. What kind of time frame would you put on us being the MMRF or one of those organizations that clearly is innovative and doing good things? >> If you go and try to give a lost drugs the traditional way you're talking about a ten to 15-year timeline which is probably more than the patients in this room would like to see. If you go the drug repurposing route the hope is there is that you can bring innovation to a patient within five years. So I would -- now, this can be sped up if patients do join hands and collaborate and work together to help make it happen. You've got physicians, scientists out there waiting to help literally that just don't have the tools and data. I was thinking last night for any disease, natural studying involving a thousand patients would be a godsend, would be something that is hard to fathom. But needless to say enormously help to scientists. The technology is available today would probably allow thousands of CFS patients to enroll and collect the data within the next few months and make it available to the scientists. So I really think whatever timeline I can talk about, it's too long. Those can be shortened dramatically by getting serious about it and I know you're serious about it but I think you need to connect maybe better than you are right now. Go to patients like me, look at the CFS community out there. There are hundreds people. We have a million people I heard CFS patients in this country. So there's many that could contribute are not contributing for various reasons. Maybe most likely I would think because they're not aware of the possibilities. That's what we theed to get better at. But I think if we do, we'll see those timelines shrink. >> This is a question for you Dr. Munoz. The question is do you have any suggestions about the networking part to bring down -- to cut across silos and work collectively? >> I think you can have networking at two levels, at the level of the scientists, physicians, and also the level of the patients. And to be effective I think those two levels need to cross pollinate with each other. There is some interesting research unfortunately it's only one study, but interesting research that was done a couple of years ago to look at rare diseases and try to find out what causes innovation to happen where it hasn't been seen before. And the conclusion there is that it takes a critical mass of physicians who are networked together and start exchanging all kinds of ideas about the diseases but it also takes a network of ideas, a network of hypotheses about particular diseases that is rich enough to allow this physician to draw from. When you're having ideas, then it starts to emerge as if by magic. That's a dynamic we need to put in motion. I think we have physicians that are involved clearly in CFS, maybe they're not as networked as they should be. Maybe they don't know each other, they don't cross pollinate enough and we've got clearly patients who are act activists out there but we've got a million people in this country who are affected by CFS just to mention patients. And we need to draw far more into these efforts than seem to be currently involved in. >> I have a number of questions here for you Suzanne. This question is you searched only for fatigue and exhaustion in adverse event database. So it's not surprising that you came up with neurotransmitters. If you looked at abnormal immune functions. Seems to me you're biassing your results by your choice of search terms. >> I'm actually not as familiar with what the adverse events database information has in there as far as the types of adverse events listed. Can you guys help me out on that >> There are thousands. Thousands of terms. There's a whole dictionary of terms. You can go in and search for others. >> So that's a very good point, and, yes, there probably is some bias to the -- to that search, but the result was pretty clear that fatigue and exhaustion was associated with the mechanism action of these types of drugs and I think that's a really good point to go back into the data and do the same type of searching for adverse events associated with other symptoms. Very good point. >> Okay. And also Suzanne, are your drug development survey results available online? >> Oh, yes. Not yet. >> But they will be >> Yeah, the responses -- we're still collecting data and then we're going to analyzing them and write them up and we'll put them out there. >> Okay. And along the same lines, there's -- this is the same thing. It's known that the quote CFS end quote literature includes studies based on overall broad and non-specific definitions. As a result, these studies include patients who don't have the disease that paishz described yefd. Did you eliminate those stoiz from the biovista analysis and if not how has that influenced your results? >> It used all information. It's 20 million abstracts and 250,000 full text articles. So it wasn't, again, the search was based on terms that are used to describe CFS and a number of biomarkers and kind of the physiologic parameters that have been used to describe CFS. So it's really a very agnostic approach to trying to find associations, tapping into all kinds of knowledge that describe the possible mechanisms that could help us explain the physiology of CFS. So it wasn't -- we didn't use a -->>: You didn't try and cull out the articles in the first place, what you did is you cast a very, very wide net. And in that process, you're going to get -- you're going to start to see conditions or symptoms, signs and symptoms coal less into groupings and that's ultimately what you found. And you found that actually there's probably more than one condition or there's more than one sub type of the condition which is what we heard yesterday, particularly from some of the clinicians, so this is a heterogeneous group of sub asbestos. >> I think maybe the way the symptoms and the signs that we use to set up the original search terms are inclusive of those that are outlined in the Canadian criteria and the international criteria. So it's very -- we tried to be as inclusive as possible. >> Melissa I have a couple here for you. But is low-grade fever a surrogate marker? >> Yeah, I don't feel comfortable commenting on specifics. And I think you have to consider the disease also when you're looking at certain outcomes and if they're appropriate measures for that. But not being a clinical expert in this area -- >> Let me see if I can take this on a little bit. So is low grade fever a surrogate marker and the question is a surrogate marker for what? For what? And when you're talking about surrogate markers you're looking at things that predict something else. And so it depends. What is the something else that it predicts? So I use the example in HIV of viral load. We know that the amount of virus over time in the blood actually predicts other kinds of illness and prolong mortality, prolongs the -- the longer you can keep the virus level low in the HIV patient, the longer the patient will live. Doctor Cox is that a fair description? So it is a -- it's a surrogate marker for mortality. That has been well established. That has been shown. So it is used -- so when we first started these studies of HIV allowing the use of viral load, we expected it probably would predict mortality down the road, something else down the road. But the companies under accelerated approval were required to show that it actually predicted that. In subsequent -- by continuing their clinical trial after the approval. Another example. Otho static hypertension. If you could show an initial effect in the clinical studies of mitadrin on blood pressure that it raised blood pressure, that you could show over time as a marker that patients clinically had fewer symptoms and just did better and there were or measures that use that, trial after trial has been done since mitadrin was approved under accelerated approval that have failed to demonstrate that that is true. Nobody can show this. It seemed so simple. And so there's been a lot of controversy about whether that drug should stay on market or come off the market under accelerated withdrawal which is part of the approval. So whether something's a surrogate marker depends on what the disease is, what you're trying to have show it as a surrogate marker of. And a great example is cholesterol. Well established that lowering cholesterol prevents heart attacks and strokes. We now approve drugs on the basis of elevated cholesterol. We don't make companies test those to demonstrate that lowering cholesterol really does do that. We just accept it. But it's a classic surrogate marker. So it depends and it's complicated, but I think -- that's what we're talking about when we're talking about accelerated approval is a marker that is predictive of something else but the key is you've gotta show after the approval that that is is in fact the case. We have just dozens of these in many fields that are involved. >> I will add that was that at some point surrogates are considered reasonably like and and are the basis for accelerated approval. When we have that confirm tore evidence we can often use surrogate that is were once used for accelerated approval for traditional approval. >> Absolutely. And we are way over time. Way over time. And we have a lot of other questions. But I'm just going to say we can't get to them. What I would like you to do is I would like you to find the presenters during lunch and ask them your questions. We're going to have just about 30 minutes for lunch, I'm sorry to say, so you might want to bring your lunch back in here for the -- in the afternoon back for the next panel, for the third panel. So thank you very much to all the panelists. (Applause) >> We'll take a very brief break. Come back quickly. (Break) >> We're going to try to get started here. We are on behind but we do want to taken full time for this panel because we feel it's very important. So unfortunately that means we'll be eating abbreviated lunch. But we have 50 minutes. So we're going to finish a little before 11:30 and then we'll have a question-and-answer period for 15 minutes. I'm going to start off and introduce myself and then go down table. You all know me I think by now. I'm Terry Michelle, a physician by training. I am currently a clinical team leader in the division of pull monary allergy. So the division of pulmonary allergy and rheumatology products is currently host division for all the chronic fatigue syndrome applications that come into the agency and I am also the agency representative. So I'll turn it over to my co moderator doctor Nancy Klimas. >> I'm a clinical immunologies in south Florida. It's been great and expediting with we're doing cool things but I won't take the time of this panel to describe all of that. But I have been taking care of patients with any CFS for more than 28 years now, and I also split my time between clinic and research and Gulf War illness related research and direct clinics in both areas so I'm veteran centric in my research. This new institute that we have is establishing both clinics and the research world and what's cool about it is that research world is right on top of of the clinic that architecturally we put the clinic in with the lab and research scientists the scientists have to walk through the waiting area of the clinic which is probably some sort of Hipaa violation, but they get to see the suffering of the patients. And it's very, very motivational for them to actually know you and see you and share your stories. I think it really keeps their eye on the target. So I'm very excited to be a part of all of that. I'm Lucinda Bateman traditionally trained general internal medicine specialist in Salt Lake City. I initially practiced primary care for about ten years but continues 2000 have had a clinic called the fatigue consultation clinic where we assess and treat patients with unexplained chronic fatigue, mostly within the spectrum of chronic fatigue syndrome and fibromyalgia. And I should add too that we also serve as center for clinical trials and research in that arm of our clinic has increased in the last five or six years. >> I'm Lisa Corbin. I God interested in taking care of patients with CFS whether they gravitated to our center for integrative medicine. My background is internal medicine I got interested in integrative medicine because my patients were asking me questions I couldn't answer. We're not trang or residents and future doctors we need to do that so that's how I got interested in the field. And I think it's a pretty natural thing for patients with CFS to gravitate to alternative medicine because we don't have any approved drugs to treat it yet. So that's why a lot of people explore alternative medicine. The last 12 years has been a journey trying to learn more and more about the CFS and trying to help patients have much better lives. >> I'm Lily Chu, a patient, I also have a background in internal medicine geriatric medicine and health services research and I'm a board member for the international association for CFS ME, a nonprofit scientific organization that many of the clingsz and researchers attending this meeting today are members of. >> I'm Jose Montoya, my background is internal medicine and infectious disease. I got into CFS after in 24 I had an encounter with one of my patients sent to me for an infectious disease possibility and I got really surprised and dismayed by the suffering that this patient had gone through and I thought that there was a possibility of an infectious agent behind the illness or at least in the -since then I have built a whole clinical and research program at Stanford that's aimed at hopefully one day being able to contribute in one way or the other to see what is the center process of CFS what are the possible infectious triggers that could lead eventually to treatments. >> I'm Chris Williams and I am a patient. I became ill in 2008. I spent 30 years in the federal government about half of that time on Capitol Hill as a health policy advisor for the senate majority leader and about the second half of my career at the agency for healthcare research and quality. Which is part of HHS. When I became ill I was still working, and I was able to work for about two and a half years with accommodations from my boss. During that time I became the representative for my agency to the chronic fatigue syndrome committee to the secretary. I now sit on the board of the CFIDs association so I am both a patient and a patient advocate. >> And I am Jennie Spotila, an attorney by training. I use that training for just over a year before I got sick in 1994. And I've been disabled since then. I got into advocacy in the late nineties and have stayed in it. I served on the board of the CFIDs association for six years, was chairman for two, and after serving that term out I started the blog occupy CFS and cover the medicine, the research, the politics and the experience of being ill. >> So thank you all for your introductions. I'm very excited about continuing the conversation that we had yesterday. So what we're hoping doop today is kind of build on that conversation and bring in the clinician viewpoint but we've also as you see, integrated patients on the panel as well because we want to make sure that we're staying on track and seeing things from a patient viewpoint. So this panel runs a little bit differently than the other panels that you've heard both yesterday and today. Panel four will run similarly this afternoon. The idea here is that we're going to have some facilitated discussion and so we're going to go to -- they have not put all of my slides in here. With that, I will read the questions to you. So question one is: What were your key take-away messages from the discussion yesterday on the most significant symptoms experienced by patients with CFS and ME? So please also describe any significant differences in your experiences as clinicians or as patients compared to yesterday's discussion. >> If I could add for the sake of time, if it's already been said by another panelist you don't have to repeat it it, you can just concur. So we're going to try to answer a lot of questions in a relatively short time. We'll going to roll down the panel starting with Cindy. >> I made a priority list on the plane of how I would answer that question and then I put it in my binder and literatured yesterday and I would say that the presentation of the patients matched my list exactly. And that would be -- I don't think I need to go through it, but this illness is dramatically limiting in terms of physical and mental stamina. It's characterized by malaise, a flare of those severe illness symptoms that involve cognitive impairment, flu-like symptoms, disturbed sleep, a whole spectrum of pain, auto nomic symptoms. I would add an important aspect is the psychological suffering these patients go through because of their illness. And this came ut the fear and the despair that comes along with this illness. The take away point I came away with from the meeting was that cognition and maybe the fear were the top on the list was the horrible suffering from this illness and the very severe limitations from the cognitive limitations being high priority. >> I concur. >> Well, yesterday my experience was very much like what many of the other patients discussed here, and to prepare for this doctor Jason and his team helped me design and analyze an online survey to answer some of the FDA's questions. We had over a thousand people responding nationally and internationally, and the responses I'm going to talk about today come from 360 to 470 responses from U.S. residents with a confirmed diagnosis and 50 percent of the population were people who did not participate in the CFIDs survey or what we submitting responses to the FDA. This is a slightly different population. But the five most significant symptoms we identified from patients were fatigue, post exertional malaise, pain, sleep, and cognitive problems and symptoms that were brought up that haven't been discussed as much in the past but as patients mentioned yesterday were also mentioned included multiple chemical or other sensitivities, gastrointestinal symptoms. These were deemed to be significant by over 50 percent of our subjects. We didn't really talk about impact yesterday in specifically but people did mention it in the discussion. What I'd like to say is that we used the standardized measure of physical functioning and found our respondents to be more disabled than 95 percent of the general U.S. population. And only 13 percent were employed with most people saying they could not work because of ME or CFS. Even for a basic activity of daily living such as personal care, 89 percent of respondents needed assistance or had to change their pre illness routine on their worst days 60 percent of respondents were bedridden and on their best days 75 percent were mostly home bound and could only do some light housework at most. There are some other things I want to mention but I'll mention that later. >> Concur with the previous panelist's observations that the story I heard yesterday exactly reflect the suffering that the patients that I see at Stanford go through. The two most salient clinical features include the cognitive dysfunction for what I have observed and the fact that happen the crashes are unpredictable. There are three observation that is I didn't hear yesterday or maybe missed them but one of them that is common for our patients when we ask them that their ability to drive their cars gets significantly impaired. And it's not a surprise because driving requires a lot of tasks what we call the perfect example of multitasking and this goats impaired. You can see when a patient it doing really bad that there's always someone with them because they're driving them f they get better, significantly better, then you start to see them coming to their visits alone. The second issue is that we have observed that there is a core of patients where herpes simplex virus, the common ordinary herpes or even the genital herpes appears to have either triggered their CFS, they say my illness started right there, or becomes the treater for the ongoing CFS illness and I'm not saying that that could be the only trigger for those patients or could be the only trigger for the whole illness. But it appears that herpes simplex alone could be a trigger or facilitator of the illness in this cohort of patients that we have that have revealed that to us and we have -- and it seems to work for that group. And the third is that it has become clear to us that in a given patient there are several triggers not just one event that either starts the disease or perpetuates the disease and you can't imagine when you want to test of efficacy of a drug how the effect of the drug could be undermined or diluted if you have several triggers happening in that patient population. So this is a fact to keep in mind when designing trials because we've noticed that lack of sleep, cognitive, emotional, or physical overload can trigger an exacerbation of CFS. If a patient is doing well on a drug because it's effective but doesn't have all these other factors controlled, including activation of simple virus like herpes, you you can see how the data could become confused in terms of drug efficacy. >> I would say I would concur with most of what was said yesterday. I think as I tried to say in a couple of informal comments, I am considered on the mild end of the spectrum. However, the mild end isn't good either. You still have a lot of significant impacts. And I think one of the things about me that is a little different is I became sick at the age of 56, which I think is well along the end of the bell curve in terms of the age of onset. And I do wonder how that is affected and how it will affect me as I age. I'm now 61 and I did not think I was going to be spending my golden years on the couch. And so I wonder how much the research may look into sort of the double whammy of this population aging and what effect that's going to have. The other issue in terms of the kinds of symptoms of concern, my biggest problem or the thing that bothers me the most is the flu-like symptoms that come back. If I have done anything and sometimes I don't know what I've done, but the flu symptoms to me are the worst thing that I have to deal with because it makes me feel really sick, and I don't feel well enough to do anything. And I think the other thing that came up yesterday, someone talked about fear versus anxiety. Fear is sort of knowing something bad is going to happen and anxiety is anticipating it might happen. I think for me, from the time I got sick to now one of my greatest anxieties was that I would get worse. And for the first couple of years I was pretty stable, and then after I retired, surprisingly enough, I did get worse. And that to me is like a big question mark. How could I have gotten worse when I stopped working? And I had all this off my plate and now I was just going to focus on my personal life and -- but it was at that point I got worse. So now I am anxious that I won't get better or that I will get even worse than I am now. So I think that the fear, the anxiety of getting worse is something I'm sure that all patients live with regardless of what level of severity they become ill. >> I thought the comments yesterday were outstanding and I just want to applaud every patient who spoke because I know it takes a lot of courage to talk about something so personal in a room full of people when who knows how many are watching on the web. So you should be applauded for that. You should feel really good about your presentations. And I think one of the things that struck me from the first panel was Dr. Kaiser talking about the working ill and as advocates we focus so much on the severely ill making sure they have a voice and can be seen but I know so many people who struggle to work and function or raise their children with this illness, and they look okay, but they go home from work at the end of the week and they collapse and we can't lose sight of these people as well because they are part of the patient community. We talked at length yesterday about the post activity collapse, trying to describe the different kinds of crashes, not being predictable. But one word that none of us used was fatigue or tired. Those are placeholders to me. Those are code words we say we're tired, we say my fatigue is bad but that's not actual what we're saying. What we're saying is the five-minute description of what a crash is and what I did the day before and how I'm going to pay for it for two weeks. Cognitive issues, people spoke very compellingly about that and all of the different domains of cognitive function that are affected. Not just concentration and memory but executive function, sdig-making, processing time, and I think that's relevant for designing trials if you want to measure whether someone is improving in cognitive function you better measure multiple dimensions in order to capture where they started and where they finished to see if there was improvement. And then also cognitive endurance is very much like physical endurance, someone spoke about having social time for an hour and 45 minutes and if you go half an hour over that, you're in really big trouble. I think Dr. Kaiser talked about going to his wife and saying we have to leave the party right now. And I know I have a look on my face that my husband can look at me and say we need to go right now. Someone spoke about the domains or the dimensions of the illness being so intertwined. So a bad night's sleep is going to make everything worse the next day. If your IBS and gut issues kick up everything else is going to be worse. It's all intertwined and that creates more challenges, I think, for designing clinical trials. And then differences, we didn't talk about gut disturbances yesterday very much. Orthostatic intolerances and he we didn't talk about how those issues can be masked. I never tested positive for any actual orthostatic intolerance issue but when I started wearing a heart rate monitor for pacing purposes, I found I was having episodes of tachycardia that I could tie to physical symptoms of episodes that I experienced and so tried some beta blockers and they improved. So there are probably lots of symptoms we have that could be measured that could be identified, we're juries not necessarily looking at them the right way. And then in terms of pain, I talked about that a little bit yesterday. Others did as well. Again, code words. Muscle pain. There's 15 different kinds of muscle pain. Where it is, what it feels like, how intense it is, does it come and go, and that's a very big issue for me. It's been a big parts of of the struggle with the illness. And not just muscle pain. Joint pain, we talked about that. Stomach pain and nerve pain. I have times where it feels like my is on fire, literally on fire. And I'm this close to going to an emergency room and what stops me is the diagnosis. Because I know I won't get treated. So there's again a lot of suffering that is going under the radar. We're not going to providers because we don't think we're going to get the care that we need in that moment. >> Thank you. That was very helpful. I'm just going to make one comment about fear and anxiety. Struck me that I spend suspended -- I've been a HIV provider forever since before the disease began. And I remember spending the first half of our HIV time with patients talking about fear and anxiety. Half the appointment was about instilling hope and getting people to hang on. And I think that's -- I feel like that's exactly what I do in my chronic fatigue practice. I spend half of my appointment trying to instill hope and getting people to hang on because I think that we are progressing in science and that there is hope to be had. But it's hard when you live in this illness to see it when you're suffering. We've all been there. Our next discussion point is what kind of clinical variables do you the panelists think the most relevant for clinical trials, what might be a valid quantifiable reliable outcome measures or endpoints for clinical trials when we evaluate future therapies. >> On our survey first I wanted to say that I think any symptom of CFS and ME could be used as a valid quantifiable and reliable outcome measure. It just depends on which scales you want to use or which tasks you want to use depending on .drug you want to test. We did ask in our survey about five objective tests commonly researched or talk about. And 66 percent of respondents had at least one abnormal result. So, for example, for natural killer cell activity which doctors Klimas is an expert on 70 percent noted an abnormal result. And our thought is tests like these could be used as study inclusion criteria as outcome mersz and a way to select sub groups for analysis. We believe that studying subgroups may yield nor effective treatments given the hetero genous nature of CFS. The second comment is that it's very important to come up with a good outcome measure for post exertional malaise. Later today Dr. Chris Snell is going to talk about that. My thought is that even though post exertion malaise has been mentioned as a system of CFS since 1994 it wasn't in my the last few years it's really been defined within the CDC and then also within the international criteria. I think when physicians think of it they think of post exertional fatigue which is not the same. Post exertional fatigue is very common in a lot of other illnesses. It's more than fatigue. Yesterday you heard people describing how they have sore throats as a precursor, the collapse, the confusion, this is a symptom that needs to be explored more in CFS and as doctor Michelle said yesterday, she was asking about timing and duration. There isn't enough basic research on post exertional malaise. The third concept is I've been learning about fatiguability from geriatric medicine, and the difference between fatigue and fatiguability is fatigue has to do with the perceived sensation of not having enough energy to do certain activities. Fatiguability not only talks about the subjective sensation of fatigue but in relation to what activities. So rather than just asking people about how tired are you, are you tired today, you're asking people about are you tired when you try to make the bed, when you're trying to brush your teeth and put it in relation to that. The reason why that's important is in the elderly we know that people self-pace, they match their activity to their energy level. It's already have in CFS if you're testing a drug and someone tells you is decreases their fatigue you have to think about is it also because they decreased their activity at the same time because if they did decrease theirtist at around the same time, that drug might have effective for fatigue but not really helping the person improve their function. Those are my comments. >> I think based on our experience it's important to try to capture aument symptoms, all the issues that affect the patients day to day lives but at the same time to carefully separate one from the other. In our experience in using certain antivirals in a group of CFS patients we have noticed that a group, and we have a paper in peer review process now for a double blinded randomized placebo control trial that those that an antiviral could be helpful in a small group of patients and in those patients we have noticed that clearly there is a cognitive improvement that occurs earlier than the physical fatigue improvement. And it was only captured when we went to our main endpoint that was the multidimensional fatigue inventory questionnaire when it was captured but one of the sub scores that emphasizes the cognitive fatigue. So it was only when we pay attention to that sub score that it became clear that there was a benefit in that trial. And you can imagine the following scenario which has occurred in our patients where if they have the early cognitive improvement, the first thing the patient wants is to do something because they feel better in that area. And you can see how that feeling of cognitive more clarity could lead to more activity navigately and then leads to a crash, et cetera. So separating the -- what constitutes fatigue, separating the physical fatigue from the cognitive fatigue could be helpful in other trials. Other aspect that we have noticed is the methods appear to matter. So if you just simply measure what happened at the end of the trial and compare that time point in your endpoint with the baseline again you may not get the statistical significance that you are pursuing because it's inherently very highly fluctuating. So patient status changes day to day, week to week, month to month. And there are statistical methods like the so called mixed effects linear regression methods that allows us to capture what's happened in all the time points or endpoint over time. When you plug the two then significant differences become evident between the placebo and the treatment groups. By the way that different between using this statistical method versus the other was the same issue that investigators in Norway faced when they analyzed their data. It became clear in the patient population was doing something when they used this statistical method that takes into account all the points in the two groups and not just the beginning and the end. And lastly, I think that in addition to carefully choosing the endpoints we have also noticed that it may be key for the design of clinical trials to keep in mind when you randomize the patients whether they have vital onset, bankrupt onset or not, the duration of of the illness. It's very hard to believe that a patient with CFS of two years' duration would have the same pathogenesis as someone with 20 years duration. So that needs to be taken into account. The female male ratio appears to be important and the severity of the disease baseline before they go into the clinical trial. Sgli want to put a big ex clamgs point on the time issue and variability of the condition. I think that's absolutely critical and I can give you an example it ties into what Dr. Munos was talking about in terms of data measurement and automatic data collection. I started wearing a fit bit in January so I went back and checked my data to tell you that my lowest day was 839 steps in 24 hours. My best day where I was really working hard was 3630 steps which is 36 percent of what's recommended for a normal person but that was my maximum effort. But that doesn't just tell you about the difference between the healthy recommendation and my max, it also shows you the huge variation in my functionality between 839 and 3630 and how dramatically different those days were. So you if only checked my steps in the first week and the last week, you missed 839 or 1100. You've got to measure that data all the way across and it will be more expensive for data analysis, but it will give you a real picture of functionality over time. >> I'm just going to concur with what others have said. >> So I'd like to concur as well. But I'd like to highlight a couple of things. One is I think it's very important to follow the symptom that's most distress to go the patient and that's also going to be the most challenging thing for research. And I was struck by what Lily mentioned about function and that's something that I think we as clinicians find very important but the functional scales out there will measure certain activities but these may not be activities that are of interest or important to the patient sitting in front of you. So, for example, I think it was one of Denise's sons who could read a book for two hours -- somebody's son couldn't read a book at all and then was able to read a book for two hours. I can guarantee there's no functional scale that's been validated that says how long can you read a book. So I think there may be some different challenges with doing CFS research that we should definitely look at function as an outcome but we may need to work on change some of the ways we measure function to be more specific for this patient population. >> I just wanted to make an a quick comment. The paper I was reading was by a national institute age program officer and he talked about measures of fatiguability. That's true that there are no validated measures of fatiguability yet. But one of the them, for example, did talk about duration of activity including cognitive activities. But that's not a validated survey yecht. Second pieces he did talk about using devices like the fit bit to look at objectively measure activity and then sort of correlate that with fatigue. Those measures are in progress. >> When I looked at this question, I answered it, there were three points I wanted to make and one was a literal answer to this question which asks what symptoms could be endpoints. And I thought that was a little bit narrow, but because I think we might not be able to use symptoms as endpoints necessarily, but so I answered that question first and I do believe that almost every major symptom this illness particularly in the priority order is quantifiable and could become a reliable outcome measure and I made a list starting with what I thought would be the most useful and I started with cognitive impairment, then the ability to remain up right or orthostatic intolerance, measures of function and measures of post exertional malaise and certainly pain and sleep are easily quantifiable and we use those tools clinically all the time to assess treatment and treatment progress in our clinic. So I think they can be translatable to endpoints for research. We also, this is the second part of the question actually, have a lot of objective measures that have been mentioned in the literature that could immediately be assessed and perhaps considered for inclusion as outcome measures and that includes all the measures of orthostatic intolerance, we have cardio pulmonary exercise testing. Immune tests that have been talked about and we have a growing literature of gene expression particularly in relation to certain activities such as a stress or exercise stressor. So all of those are going to be the potential for objective tools as outcome markers. The last thing I wanted to say is that symptoms are tricky in the measurement of as an outcome marker because as a patient's ability to function increases, they increase their function. They do more. And they'll do more to the point of -- unless you control for function in a clinical trial in other words tell people not though change their level of funding tault sometimes symptoms become -- they tell you the wrong message. And I see that naturally people increase their activity until they're limited. So those are things that need to be taken into account in the design of studies. >> And I would underscore that, that there was a very good example of a clinical trial done beautifully, eight of 24 people went back to fulltime employment from disability. Their prior air outcome vash was quality of the life and it didn't change so it was a failed clinical trial but clearly the function truly improved. So that's more and more I think function should be our primary outcome variable, not symptoms. Because patients will push to symptoms. They do it all the time every time no matter what you tell them. (Laughter). >> To say how tricky that can be, we at very early stages in our clinic used to ask the patients give us a percentage of how much you can do physically and then they would say weltz right now before starting your therapy I can only do 20 percent, and then a year later after this multiple visits there was clearly an improvement in the patient situation and then we'll ask the question again and they will say 20 percent and then you say well, but you clearly are much better than last time you said 20 percent. And what has happened in certain patients is that the 20 percent is very relatively obviously so the 20 percent at the beginning before they were clearly better was 20 percent of the world that they have adjusted and downsized. But without realizing that world had expanded to a bigger world, it was still 20 percent. So it is very tricky just if you go with certain measures. Another scale besides the MFI20 that we've found helpful that appears to track the patient's improvement is the very simple scale of the fatigue severity scale. Seems to work. >> Thank you so much. I think that is absolutely critical and I'm so glad that people brought out that point, because I think that that applies not just to chronic fatigue syndrome but it's a natural human approach to things and I know it certainly applies in other fields such as COPD where we have patients all the time who come in and you ask them how short of breath they are and they will tell you not very short of breath at all and you'll sea how many steps can you go up and they say oh, I don't go up steps anymore. I always take the elevator base I get short winded when do I that. So it's just a natural approach to things. But it's a real confounder for clinical trials that we absolutely have to take into account. So with that I think we'll move on to question No. Three. What are the key factors you take into account when making decision to prescribe or use as patients therapies to treat symptoms associated with CFS and ME. This is intent to get at some of that clinics intuition kooif piece that we heard Suzanne speaking about earlier. >> So I think the first thing that comes into mind as far as a key factor goes is the patient input. So with all patients I see whether CFS or other diseases, my initial words to them are what are they hoping to accomplish and what do they want to achieve and make sure I'm in partnership with me which for me it coordinatedally important. People come to the center, they have no interest in act cue puncture. I want want to the push it on them if it's not of interest to them but have a discussion. Bust first key factor is patient preference, their concerns and is input. Then I'm just bullet pointed my other comments for time. I would like to treat the most disabling symptom first, I like not to make too many changes at once. Sometimes I look at a patient and say oh, wow we could change this and do this and then I think that just muddies the water so much you have no idea what's helping, what's not helping and you run risk of increasing side effects and then start chasing your tail. I like to start low and go slow, recognizing that many patients with CFS have a different tolerance to medications than patients without CFS who might use the same medication. I also like especially with the pain management piece to consider retrying a medication which worked in the past so I hear from patients that a low dose of something used to help them sleep and they think it did have an impact on their pain but after a few months it quit working. And they haven't been using it in two or three years. If you can identify a treatment that worked in the past rather than just throwing it out for good I think sometimes it makes sense to revisit things that had been successful and after the body has had a chance to not see them for a while it may be successful again. I also like to try to kill two birds with one stone if possible, minimize the amount of medications so if there's a medication that may help sleep and pain, that's going to win over a separate medication for sleep and a separate medication for pain. And then I also think of each patient as being their own clinical trial. So we don't have a lot of research to guide us but we do have the patient experience and so as we're hearing about function and symptoms that becomes very important even in the one-on-one clinical trial that we're doing with our patients. I also like to be very mindful of financial and time burdens, especially with the integrative therapies that might be offered because something like acupuncture could require somebody to travel from their home, spend money. That treatment is not covered by insurance and it's not a one time deal. Three to five treatments to know whether it's going to work and if it works for you trying to lip the amount time between or trying to increase the amount of time between appointments I think is a goal. Our therapists are good at teaching people things they can go on their own. My goal is to return as much of the care of the occasion to the patient. If massage is helpful they will have a spouse come in and learn techniques. We try to be very mindful of the financial and the time burdens. And along those lines if there's a therapy covered by United States I like to highlight that what's covered. Some of the other things we've heard about physical therapy, I can't just send my patients to the therapists. They come back much, much sicker. You have to work with a physical therapist who understands the condition. Same for psychology, you can't send somebody to the psychologist who doesn't have a knowledge of CFS. You need to send someone to the psychologist who can work with them on building tools to actually improve their symptoms. >> I would say that the key factor, the primary factor I take into account is how to return a sense of control to the patient to do self-management. And this balance between quality of life and the ability to function better and take those things into account and the treatment has to be completely individualized to the patient and you have to -- it's quite important to take into consideration the risk benefit analysis every time you do an intervention and that's not only the risk in terms of safety but in terms of cost. All kinds of costs for the patient voersz whether or not it's actually an effective treatment. Many times I'll ask a patient about a medication and they'll tell me how much Ibuprofen they're taking and I'll say but does it help your pain and they'll say no it doesn't and I say why do you take it? Because I have so much pain. And it's a pain medicine. So really trying to educate patients about how to judge that risk benefit when we're doing interventions and empowering them to make those decisions day to day for all aspects of their symptoms. And then we kind of have a hierarchy of managing comorbid ities and teaching pacing and that includes activity physically and cognitively but also orthostatic component. Patients naturally do what we call a recovery position which is getting more supine and we've used an interesting measure of up right activity in our clinic that's been very helpful that up right activity is designed as feet on the floor. Defined as feet n the floor and recovery activity is when you're lying down or have your feet up. And I've discovered for most of my CFS patients the time they spend in up right activity is somewhere between two to six hours per 24 hours. Most patients who are bell adapted. And our goal is to get that higher. So we work on pacing and then primarily help the patients learn how to manage cognitive symptoms, restore are an active sleep, manage pain appropriately and orthostatic tolerance and avoid deconditioning. When we prioritize the symptoms and individualize them to the patient and try to under the best treatment for that patient it's empowering for them for selfmanagement. >> So concur with Cindy. The first priority is to see how can we prevent that it the patient is going through wild fluctuations of their disease and if there is anything that they can do to reduce the fluctuations. I used to use the words cognitive behavioral therapy and physical therapy until Lily Chu told me that that could be a misuse by some people to say that that's the treatment so we stopped using those two terms and we are now partner with a very good program in Palo Alto where patients are taught to deal with the feelings of guilt about adjusting and downsizing your life to prevent the whield fluctuations. And then as an infectious disease specialist I try to go to see if we can in a reasonable way and the most objective possible way to address the issues of infectious triggers. The very first level that we try is doing a PCR test for herpes six and EBV. We do have a small cohort of patients that are chromosomally integrated, HSV6 with vital loads of several millions in their blood and those patients are put in a special protocol, a very aggressive antiviral therapy and so those have to be done separately because it's a different nature of the disease. And then those who are PCR negative which is the vast majority, undergo a series of biological testing to see if we can in a reasonable way again see whether the trigger is HSV1, HSV2, VCV, especially for knows with significant pain in one side of their bodies, herpes six, EPV in the herpes viral world. Then according to the history carefully objected we see if there's a risk fact for -- where we have a tiny group of patients that when properly identified seemed to have responded very well to the therapy of these path owe generals. I cannot thank enough the physicians that help us with the symptom target therapy because as I've gone infectious disease my skills in the other areas has decreased so I get the help of my colleagues in the area in managing the pain, sleep issues with appropriate treatment. >> We're getting really close on time. >> I just want to make one comment that hasn't come up. And that is patient compliance. I spent 30 years in health policy and no a lot about the healthcare system, and yet as a patient, I'm not always compliant with some of the recommendations. Not necessarily the medications but the cutting back, et cetera, in fact, my doctor who's quite a character himself was at an event a few months ago and there was another one of his patients there who he introduced me to and he said, well, you know, this is my other patient who takes my advice better than you do. (Laughter). So I think that when we think about physicians making recommendations either for pharmaceutical help or pacing and other kinds of really self-management, the whole patient compliance side I think is something we have to think about and both as patients and physicians. >> Justs in terms of the risk benefit analysis and how I as a patient think about is that treatment or a medication that I want to try first thing I always ask, is there a study, a good study, on it? And the answer is usually no. So then we move on to what's the mechanism of action? Is there an indication that it will help sleep so it's worth trying and working through that. And then I will ask are there risks of serious side effects like liver toxicity or something like that. I don't even bother to ask about headache, dizziness, constipation, is it going to make me dizzy. I don't even bother to ask because I'm already dealing with all that stuff. So unless it's going to have some sort of big impact which I will find out when I try it, I don't bother. And then my risk tolerance I'm probably a little more risk averse than most patients but my risk tolerance shifts based on the amount of data, based on what we know about the drug and what we know about its mechanism of action and based on what we know about CFS patients. And so I work with my doctor to do a calculation and then start very, very low and go up very slowly and the same thing for coming off of a med. If it doesn't work coming down very, very slowly over a long period of time. And I work with a doctor who's not a CFS specialist. So my experience might be more splar to a lot of the patients who aren't here today. They work with their local doctor, maybe the person who diagnosed them, and they're not going to try all of the antivirals, they're not going to try all of of the things that the specialists are using, and I think that's a perspective we have to keep in mind. There are a lot of us working with doctors who are not trying all the latest things. So we need good data to bring to them to show it's worth trying x drug or x treatment. >> I wanted to say that it seemed like from our survey that factors that played into why patients stopped or changed a medication included side effects and cost as well as dependency and withdrawal potential of some. Sleep and pain medications out there. Another reason was decreased effectiveness over time especially with sleep medicines. For me personally, aside from just what some people are mentioned both as a patient and as a prescriber, I think about long term side effects of medications. Seems like most medicines in CFS, you have to take for a long period of time. And so I think long term side effects are worth considering. Secondly because a lot of people affected are women of child bearing age I think about the effects of medications and also about any effects on breast feeding because people are taking other medications, they have other medical conditions and they're taking or supplements, you have to think about interactions and finally I think about the experience of treating physician with a specific treatment. Especially with cutting edge treatments. >> I just wanted to make a quick statement been symptom management versus treatment of the core disorder because there's some angst about that. After my several decades of experience I want to say symptom management and the ability to do selfmanagement is really important for quality of life and to stabilize the illness to a point that we can see what the illness is. I'll also say that I think symptom management has improved the function a little bit but that the core illness remains severely disabling even with very good symptom management. So we need to have efforts on both fronts because we don't have a core treatment, we need to apply the treatments we have so that we can then move forward on core treatments. >> Can I have one last comment? >> I just wanted to emphasize also that in our survey with pacing that was considered one of the top treatments in terms of coping with day to day function. And on the other side of it exercise programs that are administered by a professional and are recommended by some medical authorities were noted by 62 percent of patients as worsening their health. This doesn't mean all exercise is bad. It means that exercise has to be done in specific ways such as taking into account orthostatic intolerance, meaning exercise lying down or sitting down and also you have to take into account the time and the frequency and intensity of the exercise. The unfortunate situation is a lot of physicians therapists other health professionals don't know enough about exercise and CFS to prescribe it in a way that's beneficial and not harmful to patients. >> Thank you very much. It was really very, very helpful. It was of a great set of comments. I'm I'll just pitch in because it didn't get mentioned that there are treatments that would enhance or modulated immune function that are also often used by various providers, myself included. We're really running out of time but we have one important final question which is what would your -- if you had a drug that you would like to explore or an intervention you'd like to explore what would be your top one or two or three choice? Just say them, not discuss them. >> My priority list were antivirals and immune modulating drugs on top. Next would be symptom management drugs for pain, stimulants for cognition, sleep meds and then medicines for orthostatic intolerance. >> I'm good with that list. >> I agree with that list. Mostly I think theimportant thing is to subgroup the subjects either by history, labs or other tests because what has been shown to be effective either in studies or in anecdotes has always been done in subgroups. It's not everyone with CFS benefit it. Intervirals, orthostatic intolerance treatments are important because it has such a huge impact on people's function and there are treatments out there for it. Someone else had mentioned in our group r. Faxomen for IBS related symptoms and >> Antivirals are primarily targeting herpes viruses and an agree with Nancy about the hoel of it's becoming obvious for many reasons that it is the immune response to the infectious agent what appears to be driving the symptoms and what we try to do by suppressing the infectious agent is to prevent the cascade that comes afterwards but in some patients it might be too late and you may have to tap into the more relation of the immune system. >> I'll concur with the rest of the panel and their suggestions >> Ditto and I would add we need a trial of retoxomad. Large scale double blind placebo controlled of that drug. If there are any products that were affect VO2 max performance please, please study them now because that I think could have great potential to improve the functionality of patients. >> Okay. So we have questions from the audience. There was an interesting question that should be addressed. And this goes to the point that there are so few expert sites. And that it's very difficult to enroll in clinical trials from a distance. Is there some way to have distance designed into clinical trials, how do we cope with access to patients and patients' access to clinical trials. There's an example. But we have right now a spouse CBT protocol that is a video group session that is done long distance and people can enroll from long distance and we designed a study that you didn't have to come to the site to participate in. That doesn't happen to be a drug intervention, but with very safe drugs for where the efficacy with be tested and the safety using distance models, I don't see why you couldn't design clinical trials with distance in mind. >> Any thoughts on that? Let's move on then. We have two questions here that ask about immune markers viral markers immune modulators and so on. You heard Lily say something like 70 percent have had natural killer cell functional study done in the survey and yet it's not in anybody's guidelines to do these things. It shows how far those of us that practice this care actually go, that we believe strong think that the biology is there, the science is there to justify these types of measures and that the measures actually compluns the care of the patients. I'll say that for myself that I'm animmunologist of course I'm going to measure the immune system and if I have a way to treat the abnormalities I have I'll try to find a way to treat those and those are certainly the clinical trials I'd be the most interested in pursuing and I'm sure with Jose same way. Might not be in the guidelines, but you have the tools to treat those things of course you'd want to measure them. So we do have a bit of a problem here, a disconnect between guidelines that feel 20 years old and the actual way we practice medicine and take care of patients. >> I think one of the messages I got from my surveys, a lot of patients mentioned they knew about these tests, natural killer cell function, repeated cardio pulmonary exam, various types of brain imaging studies, neuro psyche testing, tilt table, but weren't able to afford them. Their insurance didn't cover it or physicians didn't prescribe it because they didn't know anything about it. >> Two questions directed at Jose. One is asking if you subgroups out to foreign diseases and then other saying what's the prevalence of HSV and CMV in your practice and do you use sidovavir for HSV positive patients >> We have tried to pursue the tic born illnesses and tried through PCR antibody testing has not been hechl. However, we do have a small group of patients in whom we have reasonable tests results to believe that they can be behind their triggers and we obviously tried to address them with anti Mike crowbaral therapy. It has been hard to come up with conclusion because the numbers of patients are really small for the tick born illnesses. For herpz simplex we go with a -- more toxic and it's really hard for herpes simplex I assume that's the question, it's really hard to justify sitovovir but if we have very good evidence that hsv is causing the problem well not less at a time to use it. We use it without any doubt in transplant patients had are dying of HSV, we don't hesitate to use it. One of the things that we encounter and I hope you understand is that practicing in a medical center that is very conventional in many ways, you really have to avoid hurting any patient in trying to do good or else your program will be shut down. So it's something we're trying to follow Bernard's -- to think out of the box, to get into Bernard's box. At the same time we have to know what is the environment that we're working in. >> There's one final question that I think is a great final question for this panel. And it comes off of Suzanne's talk which is how one might be able to better pool share break down the barriers of data sharing in this group of scientists and investigators and clinicians that care for these patients. And how we might move forward in that way. Do you have any comments? >> Well, when I first heard that one of my concerns was how is that affected by certain privacy laws and also by the consent process. Because when patients consent to a study or to a registry, there usually should be some idea of who their data is going to and what the purpose of that is. And that could be a little difficult to leave it open ended. >> I think that we are in desperate need for this with a creation of a clinical trials network group for CFS. Something similar to what was done for AIDS in the '80s where within 15 years of having found that happen AIDS was causing all these issues within 15 years highly effective treatments were found and hundreds of millions of dollars with respect spent in order to arrive at that. I think that one group or one person would be very hard to make the impact that we are all looking for. It will have to come from a major effort nationwide, multi-center studies and that requires a whole infrastructure, the best groups at the FDA, statisticians, the people who have been familiar with the disease for years mostly present here and others that are not in this meeting. But it's the only way I can see making the impact that he with all desperately want. We need to have the infrastructure and that costs money. I think that would be like doing a bail out for CFS similar to the bail out that was given to the banks and all that stuff years ago. I think that's what is required to get this disease where it deserves to go. >> The CFIDs association has developed a research institute without walls, and part of the sort of the rationale or what we're trying to do is to make sure all of our researchers are connected. And I think that something like that built out own a larger scale could certainly be effective. We also have a biobank which we're gathering data from patients and also from healthy controls. So I think sort of the way that we have begun to work with our own research requiring people who get a grant from us to share their data with each other, bringing these folks together, not just people working on a single grant but bringing all of our grantees together and really making sure they are connected, it's a direction that we are moving in and perhaps there's some way to build that out in a larger way. >> That's actually what I was going to say is there's a number of people may not realize because it sounds like we're starting from scratch but these things are very, very far along. The CFIDs association has their biorepository and data set. We have the chronic fatigue initiative has a five groups that collected 200 patients, 200 controls, very sophisticated biorepository system with saliva, stool, blood, and tears collected with an excellent database. That red cap platform we used to collect that is a shareable resource through my institute, and we are happy to share if. I've already shared it with Allison's new setup in Canada so we have an international collaboration. I'd be happy to share it with others that want to -- those of you already in the study have open access to it. We all want these walls to go flying down and we're keen to do this type of sharing. I don't think we have been all that close chested with our data sets. I think we've been rushing, Suzanne set the path when she helped set up with the CDC research group and Dr. Unger and others these great big open efforts to jump into big, big data sets with the computational challenges you set up at the meetings. I think we're moving in that direction and there's data. By the way, there's another biorepose tore from the -- three of them that are multi group biorepositories and sites like ours where we have hundreds. We got freezers and we know how to store stuff correctly. So we know where we have some really amazing resources available for pharma and other scientists >> At Stanford we have a biobank of 200 CFS untreated patients, a hundred treated patients and 400 healthy controls that are also available for those who want to collaborate. >> We have a longitudinal data set 150 patients with four points in time, a biomarker discovery project and many years of other longitudinal data sets. We don't three anything away and ask permission from the get-go so we can share these resources. So there's a lot there and I think that it's an exciting time. This was a very valuable panel. >> Incredibly so. And I would like to just take this opportunity to thank all the panelists. I think there was an incredible amount of very important information that was shared here today. I need a moment to digest it all and kind of stick it in my brain. So with that we're going to break for lunch. Please be back here promptly at 12:30. We'll start panel three on time and try to keep things moving forward. Thanks, everyone. (Break) ................................................ >> We'll get going here in just a minute if folks can take their seats. Thank you. Well, thank you everybody. And welcome back from lunch. And we're starting our afternoon session, third panel. Which will be on chronic fatigue syndrome and myalgic encephalomyelitis, trial endpoints and designs. And we have a series of four speakers and then we'll have some time for questions. So hopefully folks have handy the yellow sheets for writing questions down please write them down and well collect them as we get closer to Q and A. I'll turn to my co moderator and we'll introduce ourselves. >> It's a privilege and hoerp to be here. I'm Jordan Dimitrakoff. And basically my interest in chronic fatigue syndrome myalgic encephalomyelitis emerged from my patients with chronic pain and a fair a work in the mayor of biomarkers and pain syndrome for the past ten years at Harvard Medical School. >> Thanks Jordan. And my name is Ed Cox. Sandy is my boss over there. And my background is I'm an infectious disease internal medicine trained physician and I've been at the FDA for probably ten or 12 years involved in the regulation of anti microbial products and the area of oph this. Halmic products. There are for the full spectrum of range of challenges in the area of clinical trial designs and endpoints. So it's a topic that's near and dear to my heart working through clinical trial design issues and I look forward it a series of informative talks. >> I'm Peter Rowe, I started working on CFS about 20 years ago now. I had been running a diagnostic problems clinic and we started getting patients with CFS referred to us because nobody else wanted to take care of them truthfully. And we ended up making some observations about the similarity between their symptoms and those with recurrent orthostatic intolerance problems. From there have been continuing to try to ferret out a number of clinical risk factors and different ways to treated a lessens with CFS. >> I'm Christopher Snell, an exercise scientist. I know exercise sounds like an and ath ma to CFS but I'm explain connect later on. I've been involved in CFS research for over 15 years. I got into it like a lot of people almost accidentally through actually knowing somebody with chronic fatigue syndrome. I think that's the way we really need to publicize the disease. We should have politicians adopt a CFS patient for a week and get to know them personally. (Applause). And this was a graduate student of mine who came down with chronic fatigue syndrome, a real person with the disease and said can we do something to help. And we took a look at the illness and decided we have a ready made measure of fatigue here that nobody is using, let's supply it to this population and see what happens. We never looked back from that point. So thank you. >> My name is Beth Unger and I'm currently at the center for disease control. My background and training is as an experimental and anatomic pathologist and I was at Emery university and started collaborating with a group at CDC repeated to HPV and took the opportunity to join that group because of the multidisciplinary nature of the work and the opportunity to really address public health questions and because chronic fatigue syndrome was there, they had just had a peer review and were charged with coming up with a molecular approach to understanding chronic fatigue syndrome and so that became part of my interest as well so I had the privilege of working with the group at CDC for since 1997 on CFS. >> Hi, I'm Ashley Flagell study endpoints and label development staff at CDER and my background is in measurement science and so I have developed and evaluated clinical outcome assessments across a range of conditions. And have become very interested in clinical outcome assessments for chronic fatigue syndrome because there's a real need there. And so this is been very exciting work. When I was doing my graduate work, I had the opportunity to collect some primary data and I learned during that experience that it's so important how you ask the questions in a survey or a questionnaire. And that's how I got into this. At the same time my mom had an experience where in a hospital they needed to figure out what a patient was -- what medications the patient was taking and three people had asked him what are you taking for your pawn? Knowing that he was a cancer patient with a lot of pain. And each time he said no. And then my mom said what are you using for your pain? And it turned out that he was using a very strong opioid patch that when you say the word taking, he didn't interpret it in it the way that the clinicians meant it to be interpreted. So it's critical that we find good assessments that ask the questions in the right way >> Thank you so much to all of the speakers on the panel. And interesting discussion we had in the morning and very productive suggestions that emerged from this discussion. We'll just hear from Dr. Peter Rowe about the clinical trial designs in the past looking into the future of CFS clinical trial design. >> Thank you. I appreciate the invitation from Dr. Michelle and her colleagues to be part of this symposium and have been very impressed with the effort on the part of the FDA at improving the process of making more effective therapies available for people with CFS. I've been asked to talk about clinical trials in CFS and you will be happy to know I am not review all of them. I will draw from my experience in the last 20 years as a CFS researcher and clinician and I want to address what has and hasn't worked to look at lessons from specific trials, clushg the trial in which I was most directly involved and then look at the ideal patient population for studies and I'll draw in some of these -- this section from other illnesses in which like CFS we don't really have a full grasp of the path of physiology off symptoms but have nonetheless identified effective therapies. I will talk a little bit about the ideal endpoint but others have going to be addressing that today so I'll be brief there. And then I want to end with some potential designs that might help decrease the heterogeneity of the disease we've been hearing about today. And help us identify the signal from an effective therapy from the background noise of all the the other comorbid conditions. I want to schedule out before you what my main themes are. Base CFS is so heterogeneity with variable on sets and types of symptoms for each individual and varying combinations of the comorbid conditions, we're going to need to address this and take this into account in our study designs in order to identify effective therapies and the design issues that come up the most are the careful selection of groups or subgroups which we've heard about before, decisions on which groups of patients to include at what point in the illness we include them, early or with established symptoms, we need to look at how we include people with differ levels of disease severity, and with any illness in which there's a lot of heterogeneity, another point I'm going to make is small studies are almost certainly doomed to failure from the outset. We are going to need much larger studies in order to detect clinically important but modest differences between patients. I think the outcome measures that we have available for us available now are actually fairly serviceable and while away need to choose them judiciously I want to suggest that the imperfections in measurement tools don't seem to me to be the biggest impediment to identifying effective treatments. So with that, let's look at the first of the trials in the modern era for CFS and that's the apsyche low veer study. We know CFS often follows Epstein-Barr and it has been thought that persistent symptoms of CFS might be due to Ebv. It was be used at the time on a compassion 80 basis. Those were the issues in the background for this study. There had been some preliminary observations of an improvement in symptoms. So this trial was -- had a number of design features that -- it was a randomized cross over trial. Each individual acted as his or her own control and that's a quite economical way to reduce variability between individuals in a study. There was also a clear requirement that patients undergo an evaluation at the NIH and this was a fairly extensive evaluation to confirm the diagnosis, they had to meet the 1998 or 1988 criteria for the diagnosis of the disease. And importantly the study didn't enroll an undifferentiated group with CFS, they had to have some likelihood based on their seer ology of being a candidate to improve in response to Acyclovir. Had a mean duration of I am necessary of 6.8 years. In this study each patient received IV Acyclovir or placebo and the order of treatment was andized venlgt they got it in hospital for seven days and then received 30 days of outpatient oral therapy. The outcomes included measures of their daily energy, the wellness score which was a zero to a hundred scale asking people how they felt and that was measured each day along with temperature and weekly measures of fatigue, vigor, anger, depressions and anxiety that were drawn from the profile of mood states questionnaire. Three of the 27 subjects had to be withdrawn because they developed transient reasonable failure in response the IV Acyclovir. Of the 24 who completed the study 21 rated themselves as improved during one stage of the study or the other. The interesting thing was the patient impression of improvement was evenly distributed between the 11 to felt better during the the Acyclovir phase and the ten in the placebo phase. This is the few that has the high placebo response rate and one of the interesting things is that in CFS we usually see quite a low placebo response. Anxiety, depression, those symptoms were worse during the Acyclovir period of the study. And so in the wellness store was also a bit worse. So while it wasn't a large study this trial was able to identify some adverse events of Acyclovir therapy at least in the doses used in this study, and it introduced a number of design features that I would argue we would do well to continue using including the cross over design enrich pg the study population to increase the likelihood of enrolling those who can benefit from the study drug, and insisting on a clinical observation or evaluation to confirm the diagnosis. As well as fairly simple outcome measures. Subsequent studies have evaluated a large number of interventions and a recent systematic review in 2006 showed that there had been 56 randomized trials and 14 non-randomized trials of therapy. The categories included behavioral interventions like could go sieve behavioral therapy, graded exercises tlarp and other rehabilitation strategies. Um mu known logic treatments, cord co steroids, other treatments that I've lumped in miscellaneous category, NADH, growth hormone and alternative measures. How did these treatments fa rechlt. ? The authors of the review drew three conclusions. They thought that a number of the RTC suggested that CPB may improve symptoms and improve physical function the antiviral treatments may have beneficial affects but also can be associated with harmful side effects, and their main conclusion was that most pharmaco logic treatments have not shown beneficial results. Why might that be the case? Sometimes we're dealing with wrong hypothesis, wrong drug. Sometimes the trial is negative because there's virtually no chance that it will work based on that hypothesis. It's very reasonable though given the gaps in our scientific knowledge to speculate that there are as yet unidentified mechanisms of the illness perhaps as somebody suggested yesterday even a single mechanism that explains all the symptoms of the illness and that a pharmaco logic therapy will eventually address the problem. But the other possibility is that sometimes we have the right drug but the wrong study methods and that's what I want to focus on today. We'll be look at method logic reasons for why the studies that have been conducted might have failed so that we can avoid the same errors in the future. As we heard yesterday a central challenge in streegt CFS and we heard this this morning as well, is that while patients have a common range of symptoms, there are many differences between individuals both in the types of triggers the kind of onset whether it's abrupt and the conditions. Pain, migraines, irritable bowel syndrome, painful periods, allergies, orthostatic intolerance, anxiety and depression just to name a few of the more prominent ones. This heterogeneity in the onset and the comorbid conditions creates a method logic challenge that unless the groups have approximately the same number of comorbid problems, the studies that we do risk comparing apples and oranges. The clinical heterogeneity can be reduced by careful subject selection. We've heard many comments about the case definition used for studies so we need clear case definitions and clear eligibility criteria for the subsets under study. Uneven distribution of a comorbid condition could mean an intervention is doomed from the start. So in a large study the process of randomization usually creates equal groups. You'd distribute the the -- an over representation of a prominent comorbid condition if it's untreated could overwhelm the effects of whatever the study intervention is. A second design challenge is that flare-ups in those comorbid illnesses occur with regulate. In the clinic setting, all of us who treat patients with CFS can deal with those flare-ups as they arise but the randomized clinical trial isolates one variable, the study intervention drug, and it tries to minimize the other things that you intervene with. And so that's in an attempt to isolate the signal from the therapy from the other background health problems. But you can have fluctuations in a variety of the things that people in this room have reported, for example, if endo me trio sis flares up, if you get migraines, if you're having recurrent sing co pea, all of those can effect the treatment under study. Given the heterogeneity of the disorder unless there's an incredibly powerful medication that applies across all of the heterogenious subgroups a small clinical trial will not be sufficient and we'll need much larger sample size. To illustrate the concepts I want to show some data from a cohort study we've been doing in pediatrics. We'll be presenting this next week. But in this four-year cohort we identified patients who at the beginning had what's called in a fairly clumsy medical term non-IGE mediated milk protein hyper sensitivity. And we found that 25 percent of the subjects study at baseline had this delayed milk protein problem. Their initial SF36 physical function scores were significantly different than the group that were not milk sensitive. Over the next 12 months we treated everybody with multi modal therapy and treated preliminary sensitive patients with a milk free diet and you can see they caught up to the other patients. So the statistically significant differences were no longer present on the diet. They looked like the other patients. But if you hadn't recognized that problem and the preliminary allergic patients with your clinical trial and exposed to milk on a regular basis, that can easily overwhelm the effect of any other intervention. Next I want to look at the sample sizes that have been --. Trials that have been performed thus far. We know that if you have a huge and really quite dramatic treatment effect with a drug you don't need a large study and we've seen this a few times in medicine, I remember as a trainee working in the bone marrow transplant unit of an Acyclovir had been introduced to help those patients and they were either going to live or die and the trials were not large, but that's an uncommon event in medicine. Here are some of the CFS trials done in an early phase. We talked about the Acyclovir that had just 24 people. The initial IVIG study had just 15 per group. The Terfenadine study was 15 per group and most of us as clinicians use anti his means to treat CFS patients who have allergic problems. But to think that an anti his mean might show a large enough difference in symptoms with 15 subjects per group you'd have a massive effect on their overall symptoms profile really in retrospect seems completely fanciful. And so this page, this slide has a number of studies that I think were doomed to fail. Small trials do have mrert when they identify some adverse events. So the hydro cortisone trials showed after a month or more you could induce potentially dangerous adrenal insufficiency but in general small studies risk missing the target if we're looking for a therapeutic effect. Now, contrast the small negative trials with the sample sizes used in the larger studies for a condition fibromyalgia that has a lot of similarities where CFS. It's defined by its symptoms, we lack a clear understanding of the pathophysiology. So this study of pre gabaline had one tlun in the placebo group and about the same number in each of four treatment groups with different doses. The primary outcome in this study was actually very simple. It was a daily pain scale. And with that huge sample size they were able to show a fair modest proportion of patients had more than a 30 percent improvement in their pain score. So a large sample allows you to detect a small but for some people clinically meaningful change. Here's the slide from the journal showing the differences between groups and you can see at the eight-month point it doesn't look that impressive but it was for a few people and this is an important point to take into consideration as we think about regulatory issues. Now, what about CFS studies that have had a big sample size? Here is where I need my uniformed colleagues to provide me with some help because anyone who ventures into a discussion of the pace trial probably needs an armed guard. (Laughter). But I want everybody to focus on methods and of not on their preconceptions. This study looked at specialist medical care in England and compared it to specialist medical care plus adaptive pacing, cognitive behavioral therapy and graded exercise therapy. They had 641 people and they had about 160 per group. You know already where I'm going with this. Their primary outcomes were things that we've used a lot in CFS studies. SF36 physical function score, goes from 0 to 100, a hundred being good heltd and the fatigue scale which has a score of zero to 33. There are problems with both scales, but they would normally be expected to interfere with detection of a treatment effect. The SF36 has a ceiling effect and the Chalder scale is interesting because it asks patients to rate fatigue on 11 different items ask compare that fatigue as to whether it's better or worse than usual. So the baseline comparison is with their usual function. We've heard from a lot of people who's usual function doesn't change very much so if they answer the Chalder fatigue scale they would have a very low fatigue score but it would be really quite impaired. So this is not always a very sensitive measure. It's serviceable, but it misses some things. The secondary outcomes were the clinical and global impression scale, working and social adjustment, six-minute walk, and then a variety of symptoms we have all talked about. Sleep, depression, different CFS, all relevant to improvements in general function. So despite what I would consider the potential for missing an important degree of change due to the outcome measures being a bit insensitive, the study showed small but statistically significant differences both on the fatigue scale and the SF36 physical function scale. In the group that received specialist medical care there was a three point difference on the fatigue scale and 11 percent improved by two points or more. Not really very impressive on the physical function scale 13 percent improved by eight points on a hundred point scale, again, a very modest improvement but statistically significant due to the relatively large sample size. And here's what the figure looked like when you compared all of the groups in the study. Note that the y axis is a bit truncated at the bottom and that this visually looks a bit more impressive than it actually is. I suspect most of my clinician colleagues in the room would agree with this comment and that is that if all you've been able to do with your treatment is change your fatigue score by three points on a 33 point scale over a year, that that's the kind of thing that would have made us hung up or licenses and our office headings and quit CFS in frustration. We usually do better than that. But again, I I want us to disassociate whatever our views about CBT or whether we think this trial was based on the hypothesis that CFS is largely a behavioral problem and focus on the methods lesson. This one was positive mainly bip virtue of the fact that there was a large sample size and they were able to detect a relatively small clinical difference. With that let me move on to the trial that I was a participant in. This was a two center collaboration we conducted with Steven straus where we examined the efficacy of Fludrocortisone with those with CFS. Funned in part by the CFIDs association with a grant from the NIH. The hypothesis for the study came from observations in the client clinical care of patients. A 16-year-old that was health and active until nine months before the visited to see me. She is the insidious onset of fatigue was now sleeping 12 hours a day. Awakening unrefreshed. How to lie down after showering and was much more tired after an active day. Family symptoms to everybody with experience with this illness. As a result she was unable to attend consume. She was fairly act row cyanotic, had a purple discoloration in the dependent limbs. When we subjected her to a ten-minute standing test her heart rate went up 41 beats during that time which is excessive. We then went on to a formal tilt test and that showed she reproduced her symptoms of fatigue, lightheadedness, nausea and at the 17-minute point of 70 degrees head up tilt she became her blood pressure dropped from the mid 117 over 81 town to 78 over 48 which would not allow you to remain up right very long. We diagnosed her with CFS -- and we brought her back in a month to see how things were coming along. She had had an improvement in all of her symptoms within two weeks and she resumed working at two jobs, one feeding livestock at the family farm, could spend time with friends, full school attendance, would only get tired after 45 minutes of swimming and her repeat standing tests showed an objective improvement in the heart rate variables compared to baseline. So with that as the kind of clinical background that we'd seen a few times, we went into the Florinef with these questions. Will individuals with R, well-being and objective orthostatic threatens nine weeks after starting treatment with Florinef and after starting placebo. Patients were eligibility if 18 to 50, satisfied the criteria, and undergone some evaluation to exclude other causes of chronic fatigue and these were patients in whom we could provoke hypotension during the first two stages of the up right tilt at the time. Had to have moderate severity of symptoms in order for us to detect a change but needed to be ambulatory and so they couldn't be so severely affected they were unable to walk. The study was a randomized placebo controlled double blinded study where we stratified patients by center where they were enrolled and by disease duration with the cut point at three years. We had them on Florinef and a gradual start up dose but they were on the full study dose of .1-milligram day for seven weeks, increase in fluid intakes but asked them to stick with usual sodium intake during the trial. This is what it looked like. Subjects came in were screened, had a tilt test. If they were positive they got randomized to the drug or placebo and we reepdz the tilt test or tried to get them to come back to repeat the tilt test towards the end of the treatment and again we need to be very careful when we impose objective measures which are great for science but not good for the patients as we had a fair bit of drop out on that final tilt test part of the study. The primary outcome was the percentage with the clinically important 15 point improvement in well-being as measured by the wellness score that had been used by the group in the Acyclovir trial and this asked how have you felt in the last 24 hours and they recorded a number between zero which is dying and a hundred being as good as you could expect a person to feel. And this simple little measure correlates with the SF36 and a variety of quality of life measures. The secondary outcomes were changes in different symptom scores, including the wood mental fatigue inventory, another simple one page measure of some of the cognitive dysfunction and we looked at the percent who were tolerating one further stage in their tilt test. When we calculated the sample size for this study, we assumed this 15 point improvement would be clinically meaningful and that we expected that 35 percent of those treated with Florinef would have that kind of improvement and only ten percent in the placebo group. So we came up with a sample size of a hundred, 50 per group. The results -- sorry, we enrolled a group that had a mean age of men in mid thirties, two-thirds female, with a disease duration that had a mean of more than six years and over 70 percent in the groups had been sick for more than three years. So what did we see? And the answer is not much. We were absolutely spot on with the proportion of improvements in the placebo group but far below our prediction for the Florinef group. So our formal conclusion -- no differences in any of the secondary outcomes. So our formal conclusion was that Florinef was not efficacious alone when treating dulth with CFS. We were expecting to show that this worked and that this would be available for people with CFS. And I'm -- at the time I was person who believed his data and thought if the drug didn't work I better take people off it. Maybe this was some other effect. So I went back to this girl we treated and after the randomized trial any attempt to wean her Florinef was associated with a return of really quite impressive and immediate fatigue despite a good level of conditioning. In other words, she was very fit. And so you couldn't blame the worsening on deconditioning. Her wellness scores off the drug ranged from 50 to 70. On the drug, 85 to 90. So how to reconcile a study results and the clinical observations or as I term this, with apologies to the American country music star George Jones, darling, tell me my lying eyes are wrong. (Laughter) So one potential problem with this group was that the duration of CFS was quite unlike those we see in clinic. They had a mean disease duration of more than six years. Subgroup analyses we defined before unblind being the study showed that for those who had less than three years of CFS we had a higher rate of response and for the younger patients we had a higher rate of response. But the conclusion we drew was that patients with CFS may be more refractory to treatment if they've had the illness for a long period of time. What's this tell us about the ideal patient population? And I borrowed the next couple of slides from Dr. Mark dematrack, a conference participant at a conference last October organized by Beth Unger's group and the sophisticate association and this was looking at deconstructing clinical trials. He's a psychiatrist who some of you may remember his work on low cortisol levels. He's an excellent methodologies and he has emphasized that in depression trials, past treatment reresistance influence .rate of response to a new intervention. Here's a slide from his study of vagal nerve stimulation and pharmaco tharm for depression. What you see on the bottom are number of treatments that had been failed before entry. If you failed two to three treatments your rates of response or remission were sort of in the response was in the mid 50s remission and the thirties. And the number of failed prior treatments increases you have much, much lower rates of response and remission so if you failed more than seven treatments there's no response to this therapy. And that reflects a much larger experience with the star D trials in depression. These had huge samples sizes over 2000, and they showed that if you had failed -- if you'd never had treatment of your depression or had limited treatment, the response rate to a new drug was 27 and a half percent, it dropped to 21 percent after you'd had one prior treatment failure, after two treatment failures it was down to 16 percent and so on. So he concluded in a paper he wrote in pharmaco genomics in 2006 that because CFS by definition develops of an a sdangs period of time has elapsed and as many treatment studies have obtained treatment samples based on self and clinic I guess referral, the populations under study often represent patients who are among the more refractory to any further treatment intervention. In saying that I don't think he intends and I certainly don't intend ton diminish our clil and ethical sponth p responsibilities to study those with more severe or prolong illnesses but if we're going to get drugs approved for this condition it suggests to include those individuals in our CFS trials may set the bar impossibleably high for detect ago true treatment effect and geegt these treatments on the books. At the minimum, we need to stratify by disease duration and perhaps calculate our sample sizes based on the groups that had a very short duration of illness. There are some caveat, not all of the studies have had people who have had prolonged illness. The Pace poi was a positive trial with 2.7 years duration but a couple of the ones at the bottom patients had four, eight and a half years duration and the two trials in the middle I've selected because they represent the small dextro amphetamine trials that show if the treatment sp effective, assuming these results that stand the test of a staernlg replication study, you can overcome the issue. So this is something we need to pay attention to.On to the ideal endpoint. I want to look at a couple of slides from the retuxa med study. This used a fairly simple baseline visual and log scale looking at the store of one meaning no symptoms up to ten very severe symptoms and they used a fatigue store that was calculated from the visual and log scale for fatigue post exertional worsening, need for rest and daily functioning and they did a follow up visual and log scale comparing the last two weeks versus baseline in this scale that looked at major worsening. Moderate and worsening versus no change or same range of improvements. On the right you see the fatigue score and by are the 24-week point of their intervention they were seeing significant differences in the results and when you look at the larger patient reported improvements as major moderate they had highly significant difference again, small trial needs to be replicated, but very simple trial endpoints. Others will discuss this point more, but I think some of the reasonable study endpoints would be visual and log scores for fatigue and specific CFS stms that include both frequency and the severity or impact. Fatigue scales measures to that might be specific to an outcome of interest so if you're looking at a drug that effects cognitive function maybe a questionnaire of executive function outcomes would be important. A general quality of life measure, an activity measure, we've heard a lot about numbers of steps per day, that was used in the trial in Norway that's about to be published. But other questionnaires look at activity. Functional measures and then these global clinical measures of change. I would point out that we heard from Joe yesterday that there's often a level of confusion and that creates a methods problem if we're asking people to compare how they are now versus two weeks ago. We may be asking the wrong people. And better answers may come from the clinician or a family member who's evaluating that. I also want to reflect on a clinical observation that was communicated to me by a patient. We asked this young man how he was doing. He would come back to see me after three months of an intervention and I said how are you feeling? And he said I'm about the same. And at that point his mother is rolling her eyes and hitting him in the shoulder. Ben, tell him what I did yesterday. Well, we went out and played 18 holes of golf and then came back and went to the pool for a while and then shot some hoops and went to the movies but I still feel same (laughter). And the point of that story is this guy was used to such a level of fatigue that he made the very healthy choice that I see over and over again of expanding his activity level until he generated that fatigue rather than resting so much that he had no fatigue. So we've got to watch that we don't use fatigue as the only outcome measure and I think people made that point earlier today. I want to end with some comments on potential study designs that decrease the heterogeneity in this disorder. We have to -- we've talked about sample size issues but we also have to look at more creative trial designs. And these include stratification to address the duration of illness or its severity, different subsets, we need run in periods where we treat comorbid disorders or identify responders. We need to look at randomized trials of withdrawing effective therapies at cross over designs and possibly end of one trials. So here's the structure of our current approach. We take undifferentiated CFS patients, randomize them to an intervention or placebo and then we measure the outcome. To reduce some of the heterogeneity we might try for a run in program of active, for example, if everybody got some CBT or graded exercise or specific attention to their main symptoms like allergies, migraines, that would help reduce the amount of fluctuation they were having day to day in the trial period. Now, that's probably going to be easier in countries where there are networks of specialists for CFS treatment provided that specialist treatment is actually effective. As everybody in this room knows we're pretty far from that situation here, but if we included a run in period in the design of a trial that would be very attractive to patients who would then get essentially a tune-up and then they might be more willing to join the trial, but it would also help at a method level. A second approach would be to take patients like the young woman I mentioned to you and we took her off her drug just to reassess but we could do that formally with an open -- once they've responded to a given drug, randomized them to either continue that medication or withdraw it. This has been used in seizure studies and the alternative to this is you begin a trial and you treat everybody and then after a certain point stop the treatment and then randomize the responders to that drug to either continue or withdraw the drug. Listening to the testimonials with ampligen this design to me would be much more attractive and economical as a way of testing the hypothesis that it's -- it takes a fair bit for people to stop a drug that's effective. So there are complicated issues there. Clinical trials that look at cross over designs have the chance to use each person as their own control. That assumes that there's no carry over effect of the drug in the first period so the wash out period has to be long enough. All of those designs use one enrichment strategy or another. Others are described in a monograph from the FDA that I've included on the neck slide but this -- one of the enrichment approaches would be to identify the subgroups so we expect to respond to a drug. So, for example, one that's on the horizon used in Japan for treating orthostatic intolerance, if it becomes available here, we would want to select only those who have profound or prominent orthostatic intolerance to treat in a trial with that drug. So the references to a mono graph on enrichment strategies and I've lipsed the people here and last I want to thank the people who supported our work over the last 20 years and that includes some grants on occasion from NIAID, Department of Defense and CFIDs association, we've been the recipient of an endowed chair in CFS work that's allowed me to continue on and I get help from my team as me and a volunteer parent who's been helping with the cohort study. A number of summer students and a lot of help with private funds that's kept us going. Thank you. (Applause) >> I'd like to invite caregiver Snell to the podium and he's going to talk to us about repeated cardio pulmonary exercise test results. Chris? We heard this morning that we need to come up with some new ideas to solve this problem and so I'm looking at in the audience today and one of the scary things is how many people I actually know, know well. There's a small group of very committed and hard working physicians, a slightly larger group of equally committed and long suffering patients. It's a little like being a member of an exclusive club that nobody actually wants to be in. I'm also a little embarrassed when I come here because people come up to me and say thank you for all you've done for us and I think I haven't really done anything. I have no answers. All I do is I tell people it's a real disease and I'm still trying to find out why. Preaching to the converted may be good for the ego but it's not going to move us forward. If we do that we need a new group of sinners brought into the fold that we can actually convert. A little bit of self-promotion before I start the presentation. I thought I'd change the slide actually. I put the website up there. We're no longer operating out of the University of the Pacific. The university closed the lab down without any discussion or any acknowledgment of anything that we've done in the last ten years so we've set up independently, we have an independent premises now. We have the equipment, we're dock the testing we've always been doing, we're also operating out of physicians's office including Dan's office in Tahoe. So that's where the contact is from now on. So I indicated when we started my background is exercise science. So there was never on the horizon that I would be looking at pathology. We were working with athletes, testing fire fighters for functionality. These are people that will exercise until they die on the bike. And so when we started to get involved in CFS it brought a different mind set to the picture so now we're looking at a group of people that are exercise intolerant. And we're asking them to do an exercise test. The beauty of exercise testing is it assesses function and one of the things that surprised me, nobody really had an objective measure of fatigue. Well, I'm tired, I feel feeding. How much? Well, five on the scale. So that doesn't mean anything top me. I need some hard numbers. That's not real science as far as I'm concerned. We have tests out there that we use all the time with athletes who are so concerned about their performance that they'll take the test over and own again, use it to monitor training. Interestingly they have their own off label drug trials going on that they assess through exercise testing and physical function and they get way more publicity than any drug trials involved in any mainstream illnesses let alone CFS. But it applies equally well to professional and high-level athletes as it does to people with certain pathologies. It's just a matter of asking the right questions. It has advantages. It's non-invasive. We don't have to stick a needle in anybody. I actually think it's a bioplaeshg, but we sent a paper up last year and I put biomarker in the title and one of the reviewers nearly had a fit telling me this is not a biomarker. So I added the World Health Organization definition in and it's a biomarker and they even give blood pressure as a bioplaeshg nor cardiovascular disease. A lot of the measures we use are used for different diagnosis Korean cardiovascular and pulmonary disease. So is provides diagnostic information and it evaluates an individual's capacity for dynamic exercise. You can substitute the word work for exercise if you don't like the exercise word. I just that I'm so used to using it that I've tried to train myself and I can't. It's a capacity to do work. A definition of fatigue that I like is a reduced efficiency as a result of doing work you do work, you don't recover, you're not an efficient anymore. So it's not rocket science, it's based around human physiology. We are energy producing machines. It's what we do. It's called metabolism. Uses every bodily mechanism. Everything is involved and we have a built in reserve capacity. Evolution has put that into us. Known as the fight or flight syndrome. We can find extra resources of energy whenever needed and most persons with chronic fatigue syndrome know that. If they need to do something, they can do it. Disease states reduce this capacity. Problem is in the absence of stress, that reduction is not always seen. It's like your good day. There's nothing wrong with that person, he or she looks perfectly well. I saw them out the other day riding a horse. Or they were at the fun fair or went to a game. They don't see people the next day when they're in bed and the day after that and I day after that. So exercise is an effective way to induce stress. It stresses all the systems, it will have an effect. So by stressing the system, we can start to look for differences between normal healthy individuals and individuals with a pathology. So if we only look at cardiac output at rest then we don't see any difference between those individuals. We start to stress the system and those differences start to become immediately apparent. Simple goal of exercise testing, assess the function of the cardio respiratory system. So CFS may or may not be involved with a cardio respiratory system by it's the way we produce energy. So we're looking at how efficiently that operates. And that determines your functional capacity. How much work you can do. Highly individual. We focus on an aerobic xacht. How efficiently is the body able to utilize oxygen. It's exactly the same principle that your car works on. The more efficiently your car can use oxygen, the less gasoline you use, the more energy you can produce, the greater output you get. And there are two main energy systems. And you probably heard of these systems. It's a little of a simplified way of approaching it, but essentially there's an an aerobic metabolism that means with oxygen. It is oxygen-dependent. It's highly efficient. It's time intensive so we can engage in an aerobic activity for extended peerts of time. It pre dominates at lower work loads. Sub maximal work loads we're operating aerobically with oxygen. By product carbon dioxide. And aerobic metabolism it's a misnomer to say no oxygen is needed. You don't stop breathing and you do work this way. Otherwise you would die. But essentially the energy system doesn't need oxygen to produce the energy. It contributes more at higher work loads. And the buy product of anaerobic metabolism is lactic acid. As exercise intensity increases, your capacity to produce energy an aerobically decreaseses and you start to decreasingly rely on the anaerobic energy system. Now, if you get into a debate over lactic acid with exercise physiologists, it's one of the areas that people like to disagree about. It actually is an energy substrate and particularly highly trained individuals have an extra capacity to reutilize lactic acid for the production of energy. But for most of us, it's associated with pain, reduced muscle function, altered enzyme activity, and ultimately cessation of activity. So the lactic acid will continue to build up in the bloodstream. Eventually you have to stop. Even if you don't want to. So definitions. Maximum amount of oxygen the system can deliver and combust, ie, use. Sometimes called maximum oxygen consumption. Often in research you'll see it referred to as peak oxygen consumption. It gets out of that argument about was it is really maximum but if it says peak oxygen consumption they'll give you a definition of how they evaluate peak. I'll come back to that in a little while. Anaerobic threshold is interesting because it defined most people's limits for activity. So it's the point at which you can't continue to work, you can't continue to exercise. So aerobic production is no loerng e longer sufficient to produce nshg. You have to rely on the anaerobic system, increasingly the build up of lactic acid is going to prevent you working eventually. This point is highly individual. So normal individuals, their anaerobic threshold can be anywhere between 50 or 40 to 60 percent of their maximum. Highly trained individuals I've seen numbers where endurance athletes such as tour de-France cyclists have anaerobic thresholds at 90 percent of their peak. Within ten percent of their maximum before they start to build up lactic acid. And that's partly genetics and partly adaptation to training. Adaptation to training is an important concept when you look at exercise. And really we look at that when we look at training effect, we're looking at how do people adapt to exercise. Interestingly when you look at most of the graded exercise studies doing with chronic fatigue syndrome patients even if they were done with healthy people they're not at an intensity that's going to produce a training effect. You're not going to see a training effect. So whatever effect they see has got to be something else. And it's unlikely to be quantified by any change in oxygen consumption. The topic of my talk today it endpoints for clinical trial designs. I'm going to talk about how do we measure this capacity to produce energy. Because not all measurement systems are created equal. And unfortunately people tend to think that they might be. They're certainly not all created equal economically, which very often factors into research much more than it should do. So we'll get people call us sometimes and say we're interested in the work that you're doing with exercise testing. What's it going to cost to set up? So we start to tell them and we don't think it's particularly expensive. It's 40 to $50,000 worth of equipment and you want to trained individual so you need an exercise physiologist which is really a technical capacity, they don't get paid six figures. So in the scheme of things it's not hugely expensive. But usually the neck question is is there a cheaper way of doing it? The other interesting question we get is that well, we would never get the CFS patients to do the exercise test. You might not get them to do a graded exercise program, we have no problem getting people to do exercise tests. We have a bigger problem with the sedentary controls because they want to ne what's in it for me. The CFS patients, if you explain to them well, this is going to quantify your fatigue, it validates your illness, it's useful information and they will go like an elite athlete until you have to take them off the bike. It's quite sobering to actually watch an exercise test and if we could do it we would just videotape everybody and show somebody a videotape and say look, this person is not faking it, they're not malingering. So two ways of measuring this are directly, so we do -- apologize -- a graded exercise test. That's not the same as a graded exercise program. We've had hate mail about that. You recommended graded exercise? Graded exercise tests is not the same as a graded exercise program. Or indirectly usually measured by recording a heart rate response and onset of fatigue to a graded exercise protocol. So distinction: Directly, indirectly. And there are a whole bunch of tests out there. So the easiest are when we wall field tests. These are the sorts of things you might do with your elementary school last or a group of athletes, there's the Cooper 12-minute test which essentially just measures how far you can move, how far you can traverse in 12 minutes. There's a rock port one mile fitness walking test obviously sponsored bir the shoe people so they do a timed mile walk and then the infamous 6-minute walk test. I'll come back to that. I have issues with the six-minute walk test, but it was designed for a specific purpose. It was really for cardiovascular patients that couldn't do the 12minute walk test. It's quick and dirty measure of looking at somebody's indication for cardiovascular disease. And it's reasonably accurate at predicting that. Then we have step tests, just stepping up on a box or a school, treadmill tests, the standard moving belt treadmill, and cycle ergo meter test cycling on a bike that's calibrated to provide a certain amount of information. First the field test. A group of athletes there probably does the 12-minute test and running the test. And then there's a woman in a hospital corridor. She looks like she's doing the six-minute walk test to me. They're easy to administer. Everybody likes easy. It's that big button in the ad, right? Just give me the easy button. You can test large groups of people all at once. So I can test a whole room full of people at the same time. So it's cheap. Require minimum equipment. If you really want to get fancy you can put a heart rate monitor on the person or what's everybody wearing these days, the bit -- yeah, fit bit thing. So disadvantages. We're not monitoring blood pressure and heart rate. So we've got a sick population here that might be at risk for other things such as cardiovascular disease and we're not looking at blood pressure and heart rate. Aerobic capacity. Remember that capacity to utilize oxygen, is estimated. So it's like doing your taxes. Will they accept the estimated tax or do they want an accurate calculation of how much tax you owe? May result in inadvertent max testing in some populations. This is a biggy. It's supposed to be a sub maximal test. That's why people like it. One of the issues we have with our maximal testing is that it may be unethical to do it on people that you know it's going to make sick. And I struggle with that at times. So if I could come up with a sub maximal test that worked equally well, I would be on it like white on rice. There have been studies done that have looked at cardiovascular patients where they found that over 50 percent of the cardiac patients, what they do is put portable equipment on to measure their oxygen consumption. And they found at least 50 percent of the people in some studies were actually exercising maximally compared to the other 50 percent where the six-minute walk test was a sub maximal test. I'll come back to this when we start to look at case controlled studies comparing people on a test. And we're not equating effort because we know that chronic fatigue syndrome patients won't try if you ask them to do exercise. That was meant to be a joke. (Laughter). Motivation and pacing plays a big role in the results. Motivation and pacing.Think about that. And a confirmed couch potato so I don't even have to be sick and somebody says I just want you to walk as far as you can and in six minutes. And you think, well, how far is it from my couch to the refrigerator? I've moved it actually next to the couch so I don't actually have to even get up. And so it's new territory for you. This is not even somebody who's sick. So what do you tend to do when it's new? An unknown? You back off a little bit, don't you? Let me just see how this goes. You come back in a few weeks oh, I've done this before, no big deal, you're likely to do it a little bit better. You also have to be a little careful in these studies. Who is monitoring the test? Was it somebody involved in the research or did you pull in a graduate student that hadn't turned an assignment in on time or something. I do experiments in class sometimes and I say don't give anybody any feedback while they're doing the test because it's going to alter the results. How hard is that for students to do? Virtually impossible. They've been trained all through their life to encourage people so they encourage people. So motivation and pacing play a big role in the results. Okay. So both of us will need an armed guard when we leave. Because I'm also going to talk a little bit about the pace trial. I'm going to primarily talk about the endpoints because that's what the topic of my talk is. And I'm going to talk about the only true objective measure they had in the whole study which was the six-minute walk test. So we saw that there were about 110 people in each of the treatment arms of the study. The data for the six-minute walk test is based upon 69 percent out of 110. I can't do 31 percent of 110 but 31 percent of 110 people didn't complete the six-minute walk test. Why? Baseline distance, 341 yards, I think I converted that correctly . 51-week distance, 414 yards. These are the endpoints. So it's the delta between those two that was the endpoint for the six-minute walk test. So I did a little bit more math on that because I was interested. I said well, that doesn't mean anything to me. What can I convert that to so that it gives me an indication of effect size. One of the things that we're concerned about in research is, is this effect large enough to actually be a meaningful difference. And so I converted it to miles per hour. 1.9, baseline, 2.3. Endpoint. That's not very fast. That's sort of walking around the office going to the water machine, going to get a cup of coffee. (Laughter). It's not speed walking. So I took that a little bit further. So there are tables of energy equivalents that are not particularly accurate but they're used quite often to indicate the energy costs of activities and some of the patients out here may have suffered from that, the use of these tables. That they were never developed for individual prescription. They were a research tool. And they came up with something called a metabolic equivalent. This is supposed to be based upon baseline metabolism. So they calculated everybody in the world's basal metabolism and then ampbd that and came up with a met. No, they didn't. I think they took a 40-year-old sedentary guy and calculated his metabolism and said the rest of the world's is exactly the same as that. So if you look in these tables, two miles an houry equates to about two mets, which for real scientists we can now convert that one met is worth 3.5 milliliters per kilogram per minute of oxygen. So I can double that to make seven milligrams. That would mean the patients were severely disabled and unlikely to be candidates for a heart transplant because they wouldn't survive it. So not a particularly meaningful endpoint in terms of improvement for how long the -- was it 52 weeks of graded exercise? And quite likely influenced by motivation. So the next round of tests we look at treadmill, step test, cycle ergo meter. Estimate VO2 max and anaerobic threshold from performance on field tests. You can do it by time or by heart rate. So it's indirect. Limitations may not apply top special populations, disease states, they're biased by the tested population and block heart variability of heart rate in response to exercise is enormous. American heart association don't recommend using heart rate as a indicator of exercise on an exercise test. It's not reliable. So danger of using regression equations, you have to make assumptions. So the assumptions are that there's a stead estate of heart rate achieved, there's a linear relationship between measured variable and oxygen consumption, response to any given age group is consistent, and mechanical efficiency of every individual is the same. The individual is not on medications. So there's a wealth of evidence to suggest that it may not apply in disease. Biologic variability of heart rate is well documented. It did differ by 25 percent environmental noise, elevation, time of day, illness and all affect heart rate. Tested population is going to bias it outside of normal ranges, regression equations start to break down. Okay. So I hope I have time left. Direct assessment of aerobic capacity. Maximum oxygen consumption and anaerobic threshold. We do it through gas exchange. So aerobic metabolism burns oxygen and produces carbon dioxide. We measure oxygen in, we measure carbon dioxide out. When I first started doing an it we used Douglas bags and you had a huge room with plastic bags that people breathed into and had somebody run in as soon as the bag was filled you took it over and gave them another bag and extract the gasses out of the bags and measure it. We have high tech equipment that does breath by breath analysis now that measures it in realtime. It's strongly correlated with endurance performance capability. It's dependent on cardiovascular limitations, ability of heart, hunks, and circulatory system to deliver oxygen to working muscles. Anaerobic threshold, we can measure it using gas exchange. We use -- we also get something called the respiratory exchange ratio. This is the ratio between that oxygen and carbon di oxide. It's rim the best measure of effort. We no one's that starts to get above 1.1, once the carbon dioxide starts to exceed the oxygen that person is getting close to their limits, they're getting close to maximal effort. It cannot be faked. Generally breathing during exercise is involuntary. It's extremely difficult to control and even if you did, you're not going to get an R and R above 1.1 so the test is invalidated. So indirect measurements can vary by 25 percent. We don't get ventilatory threshold. We don't get respiratory exchange ratio. We can't compare effort across individuals. How hard was this person working compared to hew hard was that person working. So determining aerobic capacity is crucial when assessing level of function, oxygen uptake and anaerobic capacity are two parameters that are closely correlated to aerobic performance. Direct measurement of aerobic capacity. Much more accurate especially in special populations in disease states. I'm going to cut to the chase a little bit now because I'm running out of time. And I'm going to highlight some of our research. Our initial round of testing use single exercise tests couldn't clearly distinguish between CFS and deconditioning. What did was the post exercise response or the post exercise morbidity. People were sick after the exercise if they were dgz with CFS generally. How do we tap into that drof off amp the test? We threw two exercise tests together. Give them a second exercise test and look at the objective measures. Our first attempt, we are saw a significant difference in oxygen consumption, CFS compared to controls. Controls usually do a little better on the second test. CFS did considerably worse. We replicated that with a larger population that first study was an NF6 for CFS. Replicated it with 51 CFS and ten controls. Slightly different results. We didn't get such a dramatic drop off in oxygen consumption, but one of the measures we did look at was workload. We get that on a bike, it's exclusive to a bike. We saw a big drop off on on second test worker load at anaerobic threshold in the CFS population that wasn't apparent in the CFS population. What that suggests that a big drop off in efficiency and utilizing oxygen post exercise so now at a much lower level of work, I have top start relying on my anaerobic energy system. So I'm starting to get less work for more effort and greater build up of metabolites. Lots of reasons why people may fail to reproduce. That's for the rest of my colleagues to find out Objective measures of fatigue in CFS ME, functional endpoint and clinical trials, better than a six-minute walk test. It reveals abnormalities across multiple systems, RER, built in measure of effort, I can compare my treatment group with my controls and say, yes, they were both working as hard, any differences must be due to the treatment. That's what we all want to be say when we run a trial of anything. Single exercise test may be insufficient to distinguish between CFS and sedentary controls. Just to finish up and just to emphasize that this is not witchcraft, there are people out there doing this, I just put cardio pulmonary into clinical trials.gov and there are just un-400 clinical trials around the world that currently include cardio pulmonary exercise testing in their protocol. And it's fairly widespread so I didn't find a continent that was not using cardio pulmonary exercise testing in a clinical trial for some purpose. Thank you. (Applause) >> Thank you, Dr. Snell. Next we'll hear from Dr. Elizabeth Unger from the Centers For Disease Control and prevention. She'll talk about the measures of chronic fatigue syndrome in a multi center clinical study that the CDC is conducting. Thank you. >> Thank you very much for the opportunity to come and participate in this workshop. I think this is a really very important opportunity to increase communication about this very important subject. So what I'm going to do today is tell you about the study that we're conducting. First of all, the background, who we're working with and what are the measures that we're collecting and why we think this is a start that will have some relevance to the question of what are good measures in this illness. You heard over and over again that physicians worldwide do recognize a condition like CFS but there are different case definitions in use and even with the same case definition there's substantial heterogeneity in the patients and it was already explained the difficulties that this can produce in clinical trials and in evaluating response to all sorts of therapies. And the factors that were already mentioned, duration of illness, the severity and types of symptoms, comorbid conditions, medications, and even basically demographics. So our study design and objectives were to really capitalize on the clinical expertise of physicians with expertise in care and treatment of CFS patients. Just as the FDA has explained for the purposes of this workshop, CFS or CFS ME is a kind of a generic term. And we asked our clinicians to enroll any patients that they considered to be CFS, CFS ME, anything that fit under this umbrella diagnosis. The goal was to collect standardized data on the major domains of illness from CFS in patients from these practices. And we thought it would be important to evaluate how much heterogeneity there are between patients and their practices, and provide some evidence and data from is it theized ways of collecting information to address the question of CFS subgroups. Our exclusion criteria were HIV positive and an age of older than 62 years at diagnosis. Probably the most important part of our study is who we have participating with us. And there are seven clinical centers. And the center in New York City, Nancy clooim's center Miami Florida and as part of the open medicine institute we have Dr. Lucinda Bateman's clinic in Utah, one in north Carolina, open medicine clinic in mountain view, California, New Jersey, and the Sierra internal medicine Incline Village doctor Danielle Peterson. So this was a contract mechanism, and CDC felt very fortunate to have these experts willing to enlist their patients to participate in our studies. The protocol was developed in collaboration with the CD but by the participating clinics and our goal was to make this fit into the clinical practice as much as possible so as to the no create a burden on the clinicians or patients. And a particularly not on the patients. The protocol was IRB approved at all participate sites and this first phase is really only cross-sectional data. Physical examination at the time of clinic or within one year was collected. And then questionnaires for self reported measures of illness were completed by the patients. A lot of the important information we're collecting is not particularly standardized but we are getting a lot of important information out of this. And this is data that we're asking permission to get from the clinic records. And the new patient intake form is scanned, whatever the clinic use to collect information on the new patients. Then of course basic demographics. Detailed medical history, the history of their presence illness, their medication lists, whatever laboratory tests have been done by the clinicians we're collecting those tests and what the results are, family history, infection and um immunization history and physical examination. While that information is not standardized, we're collecting whatever each site has and even that will be interesting to review different clinic's approaches to collecting this information. The patient self-collected instruments, the three at the top were collected at the clinic so that the patients could be monitor and supported by clinic personnel while they were completing these forms. All the other forms and I think you heard about most of these a little bit throughout the day and yesterday, were collected within a week of the clinic and a lot of them were put online so the patient could complete in a paced way as they felt they had time. I'd like to give you just an interim analysis and we've just just begun this finalizing the data. We were able to include 3 93 participants in this interim analysis. We expect a total of 450 when enrollment is really completed. And the distribution among the seven participating clinics is shown here. It's fairly evenly distributed and that's of course one of the goals of our study is to be sure we're taking a look at all seven clinics. And I'm going to go through a number of slides describing the data that we have. And in the interest of time I'm going to just emphasize the high points and also just try to give you an idea of how we expect that we will use the data once this is really finalized because this is just an interim analysis. In this slide the factors that have a little star beside them did different statistically between the clinics. And the mean age of the patients at the time of the study was 48.6 years but that did vary and the top graph. And I should emphasize that the things we find that varied statistically doesn't necessarily mean that it's a biologically meaningful different. 71 percent of the patients br female and the proportioned female in each clinic, there was a variation. What we found was 95.4 percent of the patients were white. And this of course is not really representative of CFS but this is representative of the clinic population, the clinics that we're working with. And we think this is a really good sign of access to care that we have to address in a concerted effort. The mean BMI was 27.2, 58.1 percent were married, 78 percent had greater than a college education. And 98 percent were ensured and that's another big difference from the general population. What was consistent, was that 75 percent were not working. The age at diagnosis the mean age was 38.2 years. And about two-thirds of them had a sudden onset. The range in this -- proportion that was a sudden onset was between 52 percent and 76 percent. And that did differ by the clinic. The mean duration of the illness was 15 years and the figure below there is just a -- gives a distribution of the duration of fatigue at each of the clinic sites. This slide is a compilation of different measures of fatigue that we've looked at. And one of the advantages of using multiple standardized instruments to measure the same part of the illness is to see what information we're getting from each of the instruments to see also which instruments we could possibly reduce and because we're going to be doing follow ups we're going to see which instruments give us the most reliable indication of change. So at this point this is just cross-sectional. And I'm goek emphasizing the promise instruments which are a set of instruments that were developed by NIH for use across a broad spectrum of illnesses, very highly validated and very brief. And so the promise instruments have a lot to recommend them for use if they will work in this population and they to date have not really been validated in CFS. The promise scores of fatigue was 68.3 and the scores across the clinics were different. The promise scores in fatigue. The bar graph there shows the MFI scores by clinic and MFI is scored by multiple domains. Physical fatigue is the first bar and it goes across. The lowest scores on the -- MFI and the sub score of reduced activity did differ by clinic but all of the other mif scores were similar across the clinics. And the general fatigue was the one measure that we had a big ceiling effect on. 37 percent of the patients were at the maximum score. And this was not in any of the other subscores. The promise fatigue correlated with the MFI subscales on three of the MFI subscales. The general fatigue, physical fatigue, and reduced activity. But did not correlate with well with the other two which is the reduced motivation and the mental fatigue. We have talked about how fatigue might not be actually as important as measures of function and the SF36 has been an instrument that's been used in a number of studies and what the SF36 shows very clearly again it's got a number of sub scores, you can see that the bars are all very low except for two of them. And there's -- those two relatively high scores are on vitality -- sorry, are on mental health and emotional indicating those areas of function are preserved in CFS and that again is pretty consistent across all of the clinics. We included a measure of daily activities in a vertical manner which is as described either standing or sitting with your feet on the floor and that mean level was 7.6 across the clinics. The hours of activity with your feet up horizontal was 12.8 and that did show a difference across the clinics. We asked about exercise, mild exercise, numbers of times per week that this was done, and there was a difference in this response across clinics. The average being 3.4 times a week. For measures of pain, we included the brief pain inventory and again the promise scores. And in the brief pain inventory over 80 percent of the patients had pain in the last week. And in the brief pain inventory it's only those 80 percent that go on to complete the rest of the inventory. The percent of patients taking pain medication did differ by the clinic and the severity scores in the BPI differed across combination but there was a very good correlation between the PROMISE pain interference with activity and the BPI pain interference. In measures of sleep we noted severe sleep impairment in the promise scores. In sleep questions that we had included as well as in the CDC symptom inventory. There were differences between clinics notice promise sleep related impairment and in the times awakening not rested. I'm going to somehow you a series of patterns here. And this is just a distribution of the scores on various questions that were asked in the CDC symptom inventory. And the symptom inventory score is a combination of both intensity and frequency of that symptom. And each of the clinics is a different colored bar and the various -- thisives an idea of the severity. This is the lowest to the highest. So the only thing you need to do is kind of look at the patterns and you can see that for some symptoms, it's just about everybody's over on .right where it's nor severe and as you go down each one we're seeing a shift to the left which is less severity. And most of these patterns don't different between the clinics. The ones that are have a little star, there was some difference between the clinics and these scores. But what this does show you is that for some symptoms like concentration and memory problems, there's almost a u shape where there seems one one population that that is severe function problems and another one that doesn't have any at all. So I started with those that had the highest scores moving to the least problematic at least on this measure. And you can see that the fever was the one that had the very lowest stores. We included questions from the de-Paul symptom questionnaire and that questionnaire also measures severity and frequency of the symptoms and the both the severity and frequency scores followed each other. Their scoring doesn't call for integrating them, it gives them each separate scores so in the interest of time I'm just showing you the severity scores for some of these. And interestingly the sensitivity to noise and the alcohol intolerance were the factors that showed the most difference across combination. And some of these measures such as unsteady on feet, dizziness or painting, part of the autonomic measures shows a very uneven distribution across the patients. Finally I'd like to close with just an example of how we think that the promise scores are going to help us. At the top the first line on this gives the scores that we have in our CFS patients in this study for fatigue, sleep disturbance, sleep related impairment pain interference and pain behavior and then below that there are actually it's only two studies, but five different conditions that have used these same measurements so we can make a direct comparison. Chronic pelvic pain you can see the fatigue score is less and for the promise score the t score 50 is considered average, anything above 50 is worse. And ten point differences is definitely a significant difference between them functionally. And whereas sleep is about the same in the chronic pelvic pain and pain behavior, that is, pain that's influencing what you're able to do, is approximately the same in the chronic pelvic pain as in the CFS patients in this population. Spinal cord injury, post polio syndrome, and you can see that the fatigue in these situations are all much lower. And sleep is much lower, pain is much lower. This is an interim analysis and I would like to emphasize that I think this interim analyze so far has shown us that there is a heterogeneity in the CFS population as a whole. There is a little bit between clinics but more in the patients than between clinics. This is also giving us a hint that measures themselves may be limited in their ability to distinguish robust phenotypes and/or robust subgroups, and that's why we're proposing to expand this study to some other measures. And it could very well be that other biologic correlates will be needed in order to better define subgroups. The other study -- other factor is that the data from these kinds of studies will allow us to evaluate how well these questionnaires work. For example, the MFI general fatigue scale in this population really from a specialty clinics did -- was limited by its already reaching the maximum in 40 percent of the patients. The final data sets will allow comparison of the instruments measuring the domains of CFS illness, and importantly we have follow up plan to allow the measures to correlate with changes in illness status.We have additional measures of cognition and exercise capacity that are planned in a subset of this study population, and this study is going to be expanded today include helping controls as well as ill controls and ill comparison groups are important to see how this distinguishes from other populations. Okay. So finally most important thing is to acknowledge and thank everyone involved in this study, most importantly the CFS patient study participants. They really had to fill out a lot of questions. And we understand the burden that this is and we really appreciate it. We appreciate our expert clinicians and one of the things we found most rewarding is our on site visits with each other and the clinicians also appreciate the opportunity for networking. So I think this is just a little beginning step of networking and I see more and more evidence of the need and anxiousness to start with this interacting. At the CDC I need to thank my colleagues and everyone involved in this particularly Sally Lynn and the others that are in bold who worked really, really hard to get this preliminary data organized and summarized for this session. Thank you. (Applause) >> Thanks, Beth. Now we'll hear from Ashley Slagle. She'll be talking to us about clinical outcome assessments to evaluate treatment benefit and clinical trials for CFS and ME. I'm remind folks I know we've been trying to move to keep as close to schedule as possible. If you have questions if you'll write them down we'll be collecting those and do our best to try and get some questions in while also trying to mindful of time. I would like to thank the kmps organizers for inviting me. It's critical we find effective therapies that can help CFS and ME patients significantly improve how they feel and function so to be able to participate in that effort in any way that I can is very important and I'm pleased to be here. Of course to find effective therapies, I think one of the critical steps sthap we need to be able to assess those therapies in a well-defined and reliable way. My discussion today is going to focus on some regulatory and other considerations for assessing treatment benefit or effectiveness in clinical trials of CFS and ME. So I'm not speaking on behalf of the FDA. As was mentioned I'm a fellow in the FDA working with the study endpoints and labeling development staff so the views express reside my own but are based on my experiences working during this fellowship. From the regulatory perspective, it's necessary that drug developmentalers document substantial evidence of treatment benefit from adequate and well controlled clinical trials. The regulations also specify specifically indicate that the methods of assessment of a subject's response be well defined and reliable. This is important, it means that well defined and reliable become the key criteria by which the FDA judge outcome assessments. And the evidence of treatment benefit is used to support the conclusion of effectiveness for drug approvals but also for claims that are made in labeling and promotions so communication that's provided to patients with CFS and ME. So what does this mean that outcome assessments are well defined and reliable? Well generally speaking it means measuring the right thing or the concept of interest in the right way in a defined population or targeted context of use. Specifically, it means that the score that quantifies the thing that you're measuring does so accurately and reliably and the effects that are seen in the outcome assessment can be interpreted as a clear treatment benefit. So let me define treatment benefit. FDA defines the treatment benefit as the impact of treatment on how patients feel, function, or survive. Feels is a patient's physical sensation or some per seechd mental state related tol their health related to typically daily life. Pain, weakness, cognitive problems might be -- are good examples of feelings. Function is a patient's ability to perform an activity that's a meaningful part of their daily life. Now, FDA describes three different methods that ear directly or indirectly assess treatment benefit in clinical trials. And these include measures of survival, bio markers, and clinical outcome assessments. So for the purposes of assessing treatment benefit in CFS and ME clinical trials, of course survival is an important outcome. But in order to use survival as an outcome, it would take very long clinical trials which would unnecessarily prolong the time that it takes for treatments to reach the market. So for the rest of the talk I'm going to focus on biomarkers but then spend the remainder of the discussion talking been clinical outcome assessments. Now, a biomarkers is an objective marker of biologic process path owe logic response or response to a therapeutic intervention. An example might include immune function markers. What's important to remember about biomarkers when assessing treatment benefit in clinical trials is they do not directly tell us how a patient feels, functions or survives. Instead, biomarkers may provide indirect evidence of treatment benefit. By showing a biologic treatment response but to use biomarkers as evidence of treatment benefit to support regulatory approval it would be necessary to understand how that biologic response relates to how patients feel, function and survive in their daily life. So you want to understand what's the association between or the replacement value of the indirect biomarker assessment and the directly meaningful treatment benefit. Unfortunately in CFS and ME there are no scientifically agreed upon biomarkers yet to use as outcome assessments. And remember as we heard this morning, that if treatments are approved on the basis of a biomarker that's used as a surrogate endpoint in the accelerated approval process, there's still a need to prove a true treatment benefit in subsequent trials. If we don't fully -- if that link isn't fully established yet. And if those subsequent trials fail, then the drug could be removed from the market. And so that's why it's critically important to find clil outcome assessments that can directly tell us how patients are feeling and functioning. And it's a direct evidence of treatment benefit. So let's turn our attention to clinical outcome assessments. Like I said, clinic outcome assessments may allow us the opportunity to more directly assess how patients feel and function. A clinical outcome assessment is based on a report by the patients themselves or patient reported outcome based possibly on a clinician reported outcome or another observer who doesn't need clinical training signature as parent or caregiver. Unlike a biomarker a clinical outcome assessment may be influenced by human choices, judgment, or motivation, because they depend on the cooperation, interpretation, and reporting by a person. But regardless of who is doing the reporting, all clinical outcome assessments ultimately produce a score that may be used to testify for statistical and clinically meaningful differences in trials. Clinical outcome assessments is provide critical understanding of a drug's benefits or harm and so therefore they require rug rust development and evaluation before they can be used to assess treatment benefit in clinical trials. Particularly trials that are meant to support drug approval. So only with that careful development and evaluation can one ensure the assessments are adequate validity and reliability to support claims of treatment benefit. Assessments that are reported by patients clinicians or other observers are all not all clinical outcome assessments to evaluate treatment benefit in clinical trials. There are assessments that while reported by patients or clinicians or caregivers or other observers are useful for very different purposes. They may be useful for diagnostic are purposes or, to select patients for clinical trial, used for especially deiologic sties or understanding history of a condition and oftentimes assessments that are useful for these other contexts are not really great outcome assessments in clinical trials. At least not without some modifications. So, for example, an instrument that is really great at identifying which patients have CFS or mef versus those who don't, that same instrument may be terrible at detecting treatment differences within patients in a clinical trial. One more broad point, across multiple conditions including CFS and ME I've heard concerns expressed that clinical outcome assessments must be used in conjunction with a biomarker. This isn't true. Clinical outcome assessments may be used independently in clinical trials, remember that FDA's requirement is that any assessment a well defined in reliable. The elements that are listed here will impact cl an instrument is considered well defined and reliable and I'm going to discuss each more specifically in subsequent slides. Whether a clinical outcome assessments is appropriate very much xendz on its context of use. The context of use in this case is that we're talking about the outcome assessments used in clinical trials but context of use also includes many more specific considerations. So the disease definition, which must be explicit and specific. Relevant disease characteristics, the severity level of disease, an outcome assessment that's appropriate for patients with a very severe level of disease, for example, who may be bedridden might be very different from an outcome assessment that should be used in patients who still have close to normal daily functioning. What's important is that the assessment match the population that it's being used in. The selection of an appropriate instrument also may depended on other clinical characteristics of the population, so patients with different comorbid, the demographic characteristics of the population, for example, age is very important, a self-reported assessment might work very well in adults but in children who can't report for themselves we couldn't use the same outcome assessment. The context of use considerations also include the study, the study design, the other endpoints being used and how this clinical outcome assessments will fit in with those other endpoints and then of course the type of labeling claims that are being sought. One of the biggest challenges in CFS and ME related to the context of use is that the disease definition isn't entirely clear. But there are ways around that. What is important is to identify a rationale set of clinical trial entry criteria. And being careful to exclude other conditions to the extent possible. Of course in clinical practice, many other comorbid exist but it's important to have as pure of a population in your clinical trial as possible to limit any unwanted variability that might obscure treatment effects. Of course, there is a need to identify the most precisely defined population, but researchers and the FDA recognize that patient selection really needs to be practical too so that you can recruit sufficient patients. There are a number of potential sub populations within CFS and ME and I've listed several here, but we know that there are more. You've probably realized by now the things we want to measure the concepts we want to measure and the context of use go hand in hand. We can't consider one without considering the other. So it's critical to define your context of use, define what concepts are important to measure, and then select your outcome measure. And actually yesterday's one of the goals of yesterday's meetings was to help us really start to better understand what are the important things that we should be measuring in different populations of patients with varying experiences. In thinking about the things that may be important to assess in clinical trials I've listed here fairly comprehensive list of symptoms. Of course, not all patients experience all of these symptoms. And this list doesn't reflect all of the things that all patients experience. It also focuses primarily on symptoms and doesn't reflect some of the more multi factorial experiences that patients have. Family concerns, social concerns, financial impacts, and general quality of life. Question know all of these things are important. But these impacts are very difficult to assess in clinical trials, particularly because they're multi factorial and we heard an example this morning of a clinical trial that failed although the patients they were getting better, they were going back to work, the outcome assessment assessed quality of life which was probably not the best measure to use and so you don't know when a treatment fails if it's the drug really not effective or was the outcome assessment that you used not the appropriate one. So the goal is to identify the experiences that are important to patients in the various sub populations, identify those things that are important and can be feasibly and accurately measured in a clinical trial setting, and identify those things that you would hope to see a drug therapy have some impact on. Then find the outcome assessments to measure those things. And again, yesterday was a great start at generating that list of things that we should be thinking about measuring in clinical trials. So I've tried to represent visually here how to think about what to select when you're measuring something in a clinical trial. So the yellow circle represents all of the things that the broad group of CFS and ME patients might experience. The green circle represents a particular well-defined subpopulation of patients who, for example, experience post exertional fatigue or may lays. The blue circle represents the effects of a drug treatment. Where the green and the blue circles overlap represents the right thing to assess in a clinical trial of this particular subpopulation in order to document treatment benefit. Now, in this slide we have all of the original circles that I described but we also have the addition of this rust colored circle. It represents the assessment of something that's not experienced by or relevant to the subpopulation that was in this particular trial. Even though it's relevant to the broader population, and it may do a good job of distinguishing between your clinical trial participants and those who shouldn't be in the clinical trial, this would not pick up a treatment benefit in the clinical trial of that population if you were to measure those things. So again, it's key to define the right things and good measures of those things up front so you don't have to look back on trials and wonder if it's truly the drug failing or if it's your outcome assessment that's failing. Because CFS and ME are highly symptomatic conditions that serious impact functions and daily life I'm going to discuss some issues that are specific to patient reported outcome measures. Here's a specific example highlighting a concern of the content of a patient reported outcome measure. So say, for example, an investigator intenth to assess a study population of primarily bedridden patients where minimal physical activity is very difficult. So the concept of interest or what the thing that we would want to measure is physical functioning, we want to see if patients are able to improve their physical activities. So an investigator could look for an assessment that assesses physical functioning, makes sense. But if you look closely at the assessment, and this is based on an actual example that happened in another condition, you see in the items that there's a question do you have trouble running to the bus? So it says it's assessing physical functioning which you want to assess but the items assessing that are not appropriate. Clearly this would not be appropriate for bedridden population. This is why it's so important to consider every specific item or question in a questionnaire. And how those items contribute to the domain scores and then the overall score of the instrument. So to avoid using questionnaires that don't appropriately target the population, the FDA would look at individual items to make up those scores to determine if the items and the instrument are appropriate in that context of use. In this case the colored boxes represent scores that are turned into endpoints in a clinical trial. As an example if it the overall concept to be measured is fatigue, then it's important to consider what items and domains make up fatigue. Domain A might include items that produce a domain score that relates to physical tiredness. While domain B might represent mental tiredness. There could be a domain C that represents emotional tiredness. There are or domains of fatigue that possibly should be included. This is where it is critical to talk to patients in the intended population to really fully understand what are the meaningful aspects of a broader concept that we should be measuring in clinical trials to understand treatment benefit. So there's some other considerations for selecting clinical outcome assessments for use in clinical trials. And one is the recall period. When know that people have difficulty recalling or averaging experiences over long periods of time. For symptoms that vary over the course of a day or even hours, asking a patient to recall over several weeks is unlikely to give us very reliable results. And I think this is particularly important in patients who are having some cognitive activities. It makes it even more difficult to think about thinking about your experience over the last week, two weeks, 30 days. The length of a questionnaire is also important to consider. Investigators need to think about responder burden. Unlike some studies where patients are completing assessments just one time, in a clinical trial assessments are completed multiple times. Sometimes daily. And so lengthy questionnaires really don't lend themselves to be being used in clinical trials with these repeated assessments. And again, thinking about the symptom that we have heard a lot over the last two days is that patients who are having cognitive difficulties and sort of hit a wall after reading for so many minutes if they're trying to fill out a very long questionnaire, it may become impossible after just a few questions. The mode of administration should given some consideration. Electronic data capture does help to limit missing data, but you have to be careful. If an instrument was developed for paper administration if you just transfer it onto an electronic platform, you have to be careful that you haven't change the way patients are interpreting those questions and you don't want it to impact how patients respond to that assessment. And then once you've assured content validity, the Roman numeral period, the length of the questionnaire, the mode of administration is established, then it's important to evaluate the other psycho met Rick measurement properties of an instrument and this includes reliability, construct validity and the ability to detect change. Now I'm going to turn ourn attention to talking about some existing instruments. This slide identifies just some of the many patient reported outcome assessments that have been used in CFS and ME. Certainly doesn't represent all of them. What this slide does highlight is that there are already quite a few existing instruments that have been identified which represent the many different symptoms and domains that may be relevant to various sub populations within CFS and ME. However, many of these have been developed for purposes other than that as outcome assessments in clinical trials where they have not been specifically developed for CFS and ME subpopulations. So they need to be evaluated carefully. And potentially modified before they're used in clinical trials. Snadz to patient reported outcomes assessments there are also clinician reported outcome assessments and observer reported outcome assessments. As listed a couple of clinician reported outcome assessments here, and so far we haven't found any observer reported assessments that may be useful for assessing experiences in young children or other people who are unable to report for themselves. So I'd like to point out some of the challenges we've identified in existing instruments when we consider them for use as outcome assessments in clinical trials. And I don't want to pick apart specific instruments. That's not my goal. But I hope that by identifying some of these broader concerns that it can help guide instrument selection for clinical trials. So there are some instruments and the performance scale is an example of this, that have been developed for diagnostic purposes or as a classification tool. These types of tools rarely can capture the appropriate concepts that are relevant to treatment benefit. Some of the assessments have been developed as especially deem logic benefits so they're very comprehensive which is great for that kind of study but they're too long for use in clinical trials, especially with repeated administration. The recall period is often way too long for patients to report accurately, and they include a lot of symptoms that not everyone experiences. There have been some generic measures that have been developed for a broad population and they're not specific to CFS and ME. Concerns here are that these measures include items that aren't relevant at all to the population, for example, the can you run to the -- how much trouble do you have running to the bus -- or they might be missing some items that are very important to measure in this population. In some cases that are conceptual framework concerns, for example, checklists where you're checking yes or no whether you have a symptom or not. But there's no conceptual framework or scoring algorithm that's available to produce a score that can support an endpoint. I see also problems with conceptual framework issues in general measures of fatigue where items ask about fatigue generally but they don't consider including items within the various domains of fatigue that are relevant to CFS and ME patients. And in some cases the measures don't account for activity level which we're hearing is very important to consider when you're thinking about symptoms in CFS and ME. There are also concerns with individual items and some of these measures in some cases the items are very confusing where they ask one item will ask about trouble falling asleep or troubling sleeping too much, which leaves patients unsure how to respond to that. The items may be too general, sometimes they ask about are your signs or symptoms of disease present. Well, what signs or symptoms of disease? And then in other cases some of the items are just not really relevant and don't make a lot of sense or patients don't know how to interpret them. So items like I feel fit or I feel peppy are not really clear or relevant. So of the concerns that I highlighted in my last slide, of will impact whether an instrument is well twiend or reliable. And unfortunately none of the instruments I've reviewed so far are ideal to assess treatment benefit in clinical trials of CFS and ME patients. But it's important not to let the perfect be en any of the good. The ideal option is to continue looking and be find the existing measures that are appropriate within specific subpopulations. But if this is impossible, another really good option is to modify existing instruments to make them well defined and reliable for a particular clinical trial. And there are many instruments that are already existing that we can consider to modify. And we can remove the problems that we already know exist in some of these instruments. And then of course a final option is to develop a new instrument. But with all of the work that's already been put into. These existing instruments, all that we know, and we can continue to learn about the CFS and ME population, I don't think that developing a new measure is necessary. As we think about modifying or developing instruments for use in CFS and ME I would like to describe a process through which the FDA is able to work with the public to do this. This process is called the drug development tool qualification process and it's outside of a specific drug development program. So what this allows is for various groups to work together and come to the FDA through this process and receive consultation and advice from the FDA on the instrument that they're putting together for use in the future clinical trials and the idea is that these instruments would be available publicly and could be used across many clinical trials within the same context of use. I think this is a really good approach to instrument development or modification because the FDA is participating, various groups are coming together with a lot of different expertise, and it helps to reduce scientific uncertainty and risk to the pharmaceutical companies ultimately who would use these outcome assessments in their clinical trials. And I've provided some links here with some additional information and an email address if you're interested in finding out more information about the DDT qualification process. Thank you. >> We'll take some questions and also be watching the clock too. >> I have the sense that this panel actually deserves a -- I do think that the talks are quite productive and insightful. Probably start with a question for anyone on the panel. The question basically is it's clear that we need larger clinical trials because of the heterogeneity of ME, but those big trials are usually paid for by pharma who Dr. Munos told us won't fund because of how undefine the disease is. Comments from the panel? >> I think this is a big problem. Others had commented that we need something like what was available for the HIV network or what we see being available for patients with pediatric cancer. Almost every patient that shows up in our institution is slotted about a multi centered trial. That requires some sort of effort, I'm not sure where it would come from, when we submitted our first grant to the NIH for the study of fror it had a priority score of 326 which my colleagues emgz ld was you're dead and buried and I think the reason the scoring got overturned was that we were able to say your sgernl intramural researcher wants to work on this, how do you explain the discrepancy between that vote of confidence and the review committee. And then a second problem we had was when we were half which through the Florinef trial we proposed studying another drug in a cross over trial and the review committee didn't give it a score because there was no mechanism for funding clinical trials at the NIH since we were currently being funded for a clinical trial by the NIH I was I was a little bit puzzled by that but in the end I think that comment was correct that the first trial was addressing path owe physiologic mechanisms and the NIH focuses on that so they're not a clinical trials agency for the most part. I think that's a big gap that we're struggling with is that if studies don't come from pharma, I don't know who would fund them. >> Clearly because it's a multidimensional illness and the endpoints are likely to rely on expertise from different individuals coordinating the whole thing is a major task and a major economic problem. So, yes, it needs something to drive it. >> Ashley? >> I think that one of the things that we can do is try to encourage pharmaceutical companies to complete these trials. In my world one of those things we can do is to develop good outcome assessments and so that there's lower risk to the pharmaceutical companies in conduct being these trials. If they know owe know what they're shooting for and have well established measures that they know that they can use that have been developed in consultation with the FDA, then I hope that they would be more likely to engage in clinical study >> It goes back to what was in the first panel, reduce being the risk for the drug companies and showing once we get a critical mass of data and infrastructure, then they will come, that's -- I believe that that's true. And just CDC doesn't do clinical trials, but we're supporting it by trying to make some of the first data, a start on the data and a construct. >> I we actually had some discussion with some other colleagues during the lunch break and I think one of the very promising mechanisms for doing this is actually through a partnership through the NIH so there is a partnership grant program it's an RFA which actually provides researchers with access to the NIH clinical center resources. So it's a shared grant mechanism which allows people to do clinical trials utilizing the NIH clil center research. So that might be promising or to what Dr. Rowe was doing back in the day with Dr. Straus. That's something that could be potentially a good start. >> All right. The next question here is asking or -- 18 percent of respondents diagnosed with ME CFS have only that diagnosis the high rate of co more bit at this maybe an additional challenge. It's asking if there's any suggestions from the panel about how this challenge might be addressed with regards to clinical trials. Dr. Rowe do you want to start off? >> One option might be to do what they've done in fibromyalgia where they faced the issue with multi co more bit at thises and I think parted is you have to have a huge number sample size that there is a random distribution of the comorbid problems. That's one option. The other is that any two people with the illness next to one another have a lot of differences and so using each as his or her own control is another way of getting around that problem. >> Just trying to to be respectful to all the people that ask the questions given the limit time that's why we're trying to probably address some more questions. There's a question for doctor Unger. Given the results from the CDC trial. The question basically says that you mentioned that infection slash I am mu anization is in your database. Have you looked at a connection between immunization or exacerbation of symptoms and are the data maintained in such a way that such an analysis could be done? >> We don't have onset specifically correlated with the infections or immunizations but this is our first clans to look at the data in this way so from that first collection of information we decide what additional questions and how to go forward in the next design. And that was not -- because that wasn't a standardized kind of question the data cleaning up the data so to the point where we can analyze is taking us a bit longer but we'll have that information. At a first look there are interesting differences in numbers of infections and immunizations. We think this will be an important data point but it will only be the beginning of looking at it. >> A question for Ashley. Asking about at the time that a study is being designed does the FDA assist with the development of the trial and also coming to agreement on outcome measures? And it's essentially just asking does the FDA advise. Feel free and happy to send it to me if you want to. >> There are two processes. At least for developing outcome assessments. You can think about going through the. ND or NDA process where you are develop a specific drug product and going through the process with the FDA and in that case during that, the course of normal meetings you would of course if you're bringing information to the FDA about your clinical outcome assessments you will receive a response. You're likely to receive a response. The other option is to go through this qualification process that I mentioned that's outside of the drug development a specific drug development process and then the FDA is specifically providing consultation to the developers of that instrument and then ultimately once it's qualified, that stument could be used in multiple clinical trials. Dr. Cox? >> All good points. Just sort of on a broader level just thinking about this topic, when a clinical trial is being designed when an endpoint is being selected, we welcome the opportunity to talk with those that are designing the trial and think it's very important step in the clinical trial design. So for those that are designing trials for those that are designing endpoints that they're going to use in trials please do. Come talk too us at the point that you're designing concur trial. We welcome the opportunity to engage at that point and which it's important for us to do so. >> Can say one more thing? The earlier the better. When you're designing assessments the earlier you start talking to the FDA about it, the better off you are. Pre IND phase. Might be a little bit early buff the earlier the better. >> What's substantial improvement from baseline in ZO2 maximum and in steps walked? >> VO2 you would expect to see progressive improvement with the training effect. Given that we don't have a lot of data that might show a treatment effect for something other than training, so the real problem is that if there's a VO2 deficit we don't know what's causing it so if it's an immune problem, then if you fix the problem and all of a sudden the VO2 goes up, you could use a number of different criteria. There are different disability categories rated by oxygen consumption. So if somebody moved from severely disabled to moderately disabled, that would seem to be a reasonable way of looking at it. Five percent improvement, ten percent improvement, and relate that to functionality. Then that would be another way of looking at it. What you'd expect to see with an athlete is you're going to see progressive improvement over time up to a ceiling. But it's fairly dramatic. You'll see big improvements in VO2 from training in two weeks. As for the steps, we don't have criteria for that. It's not -- it's not necessarily helpful unless you decide that it's related to something else that there's not a lot of data to show how much -- how many steps you're going to get. There's probably data for the six-minute walk test in terms of distance and the way they grade cardiac disease but does that apply to chronic fatigue syndrome? I don't know. You need specific data for the population for a test like that to see where the baseline is. >> We'll go for another five minutes with everyone's permission. >> One more quick question for Chris Snell. It's asking about insurance coverage or payment for exercise testing. Is that something that in your experience -->>: We don't third party bill, but we advise anybody we see in the clinic to send it into the insurance company and the insurance company usually copies ups the letter when they pay people and we get a reasonable response to people getting paid. >> Underlying diagnoses -->>: Somebody asked me that earlier and I can't remember. We actually give they have the code number. So I'll look it up. >> Great. Thank you. So there are two questions. I just picked a couple. One of them is Dr. Unger and the other one is, the question is FDA says patient population must be well defined for clinical trials yet CDC study doesn't ask participate clinics to use any particular definition. How do you reconcile these two views of how to do trials? >> So ours isn't a treatment trial and we're not -- so we're collecting data on what CFS ME CFS looks like in the clinics, what the clinicians who treat these patients, what they look like. That objective data can then be used to drive a case definition of more or less specificity depending on the use and the indication. And there are times when you would want a very broad case definition and then there are times when you would want a restrict it. For example, in the study he required postural hypotension because he was trying to intervene on that. That's a different use of a case definition. That I think is the distinction. >> Thank you. Just a question again to Dr. Hagel you pointed out the problems with various used outcome measures. You say that the instruments respect not ideal. Does this mean we can't do trials because we have no instruments until we modify the existing instruments or develop new ones? >> I don't think -- it shouldn't hold up progress on trials. I think that not having great instruments does add bladed more risk to the trial. There may be a treatment benefit that's there that's not able to be picked up by the instruments that are used. If there's a huge treatment effect, then even a very broad instrument that may not be specific enough can probably detect that treatment. So I think matching the instrument to the population and measuring the right thing as best as you can even finance it's not ideal is still an appropriate way to move forward. But we should also be working to modify instruments so that they can be more -- better defined and more reliable. >> The essence of the question asks about what we -- knowledge that might be gained from brain mapping and the impact that might have on studies in patients with ME CFS. Any of thoughts on that from the panel? It's a broad question. >> Brain mapping of the patients or researchers? (Laughter). >> It's the general research. Any thoughts. It's a big question. >> Here's a question. Differences in racial and ethnic groups and the refuse lens of CFS. If anyone can comment on that. >> From CDC's population based studies CFS is very common in racial ethnic minorities, and it's of equal or increased prevalence. So it depends a little bit on how the study was done. So just going back to the brain mapping, I think this is the future. There's going to be a lot of data that's coming from brain imaging studies, New England Journal of medicine published something where they could measure pain by functional magnetic -- MRI, so I think this will eventually have an impact. It may be a little bit too son. And the interesting thing is studies like that and the gene profiling, you end up going very deep ask getting a lot of data on a very few patients. And that's one approach and you're probably going to get something there, but given the heterogeneity of the illness we also have to go broad and so that we can go deep and make sure that when we go deep we have a uniform group that we're going deep on. And I think some of the confusion in the literature has been on these different comorbidities and stages of the illness. >> Building on what you said to add up to the MRI studies. I think it might also be important in the future to use the paradigm, you might need to stress the system so to say just to baseline evaluation or a single measurement might not be ideal and we night need to follow people. >> CDC and other people have definitely found that the difference at baseline are very different from after putting the disease in motion and responding to all kinds of stresses, exercise, cognitive, infections. >> We would like to thing our speakers for a serious of excellent presentations and again audience for all your questions. I think we will have to move to the break so we'll take a 14-minute break. We apologize to those those whose questions we did not get to. (Break) >> Good afternoon. Good afternoon. Let's get started again, please. Panel No. Four. Thank you. My name is Dennis Nangan, the moderator for the final panel meeting and we're going to be doing a summary and a path forward the point of this session of course is to do a little recap of what we've harder, make sure we heard what we thought we heard, and then to be able to document this and to be able to pass it along to the FDA. And to the other federal agencies and essential everybody. My name is Dennis Mangan I'm a former NIH employee. I was a chair of the trans NIH working group ME CFS research working group. And now I'm sort of retired but I'm a passionate advocate for research on chronic fatigue syndrome and other medical illnesses. My co moderator today is >> Good afternoon. My affiliation is up there. I'm at the FDA and a colleague of doctor Terry Michelle who has been very instrumental in putting this symposium and working hard on the background and also for these two days. My introduction to the chronic fatigue syndrome has been with within the FDA where all the drugs for this disease were transferred to our division and I've been involved in this for the last couple years and working hard and hopefully we'll see some drugs coming down the pike ultimately leading to approval for this devastating disease. What we'll do briefly here is introduce ourselves which I just did, and pass down the table here for those folks who are new to introduce themselves and for those who have been introduced previously perhaps just to say hello and move on and go through the questions. So Dr. Chu, if you can brief introduce yourself. You have been in the panel up here before. >> Yes. So just to remind everyone I'm a patient and also a physician and board member of the -->>: Jord done Dimitrakoff from Boston, urologist seeing patients with chronic pain syndrome and have developed an interest in seeing patients with chronic fatigue syndrome as well as learning more about the pathophysiology and treatments for chronic fatigue syndrome. >> I'm Nancy Klimas from south Florida and I'm a clinician in and an investigator and I'd be curious how many people from pharma are in the room. Very good. Thank you. >> And I'm Nancy Lee, I am the designated federal officer for the chronic fatigue syndrome advisory committee the director of the office on women's health at the Department of Health and Human Services. What I know about chronic fatigue syndrome is probably what I have learned in my two years as DFO so I'm in no way an expert but I have been with the federal government for about 25 years, I was at CDC as a medical epdemiolojist and now I'm at the department and I'm an internist by training. I'm Susan my and I'm the new Dennis Mangan at NIH. Working group a lot of words, but we're a coordinated working group, we have members from almost every institute and center on that working group and we are going to become the face of ME CFS at NIH. And I'll leave it there and safe the comments for later. >> Robert Miller, I'm a ME CFS patient and also an ampligen patient. I've had ME CFS since 1982 and I've been on the drug since 2000 on and often and very happy to be here. Thank you. >> I'm Terry Michelle I think probably everyone here knows me by now. >> Jody Roth, from Eli Lily and company, director of regulatory affairs for biomedicines group and I appreciate the opportunity to be here. I've been in pharma over 19 years and hope to bring some of the experience and discussion around what it might take to get into this indication. >> Thanks to the panel members for taking time out of their schedules to be here and to participate in this panel discussion. Again, like panel two, we're going to have a series of questions that we're going to pose to the panelists and down the table here and ask them to give their impressions of what they've heard over the past two days and the first question that we're going to ask is what were the key messages on drug development that you heard at this meeting over the past two days. I'll start with Dr. Chu. >> I heard two of the main messages that I received was that first, there needs to be more data early on the first panel, and I agree with him. I think there's a lot of even basic information on CFS that's not been well researched yet. I do think, however, that patient organizations have tried very hard to raise money and also to collect this data. But we need the support of the federal government to invest in the research for this. In both the pafk and clinical research. The data out there is about $6 million a year is being spend on CFS research. That's very much in disproportion to the 18 to 24 billion annually that's lost in both direct and indirect costs due to this disease. We keep hearing in past years that there are not enough young investigators who put in grant application for CFS. And I think in part that's because for the last 20 years there hasn't been that much of a great support. Used to be an investigator and I got there because I had mentors I knew there was funding availability. I I can see why there would not be a huge pipeline of junior investigators and that's partly because they don't see their senior investigators if they have any role models getting the fund they needed. That's .1. Two is about the open access data that people have mentioned. Aside from the fact that there was of a question during my panel about how do you access trials if you're not in the same geographical area as a center I want to talk about home bound patients. I used to visit people at home, and there was a huge difference between the patients I saw in clinic versus the ones I saw when I visited them at home. And right now practically all studies of CFS with the exception of two I've heard about have been in clinics and not based outside of clinics. There are things that can be done outside off clinics like blood draws, ultrasounds, et cetera, some of those are available that weren't available in the past. So that's two points I wanted to make. >> I think one of the -- there are a couple of messages for me. Number one message for me during this meeting is that it is actually possible to have a drug for ME CFS and I think that's very encouraging. I think this is good news for the patients, for the researchers, for the clinicians trying to help patients with ME CFS and I think this is really, really really optimistic and good news. The number two message for me is that I think that we would all agree that there's a great deal of support and enthusiasm from the FDA from the federal government supporting the development and accelerating the development and potentially approval of a medication or medications for ME CFS and I think for this we all have to applaud Dr. Michelle who helped put together this meeting and I think this is really a great message for the community. (Applause) Thank you again. And everyone the FDA and everyone actually being here. Thank you to the patients for being here. (Applause). You probably deserve most of our applause and for the clinicians and research expenditures the drug companies that are represented here. The third message just listening to Dr. Chu is actually something that we have been discussing over the past year. I'm involved as a member of the chronic fatigue syndrome advisory committee at the Department of Health and Human Services where I'm actually very fortunate to work with both Dr. Michelle, doctor Nancy Lee and Dr. Suzanne Meir and we've been having a discussion over the past year about young investigators and bring them to the field. I think this meeting actually validates the importance of this discussion. The importance of how important -- sorry for the repetition -- it is for young people to get involved in ME CFS at the very early stage of their career. We've been discussing different mechanisms of providing young investigators with support early in their career and also trying to retain established investigators in the field of ME CFS. A lot of those discussions have been tainted by pessimism actually and by the fact that people don't see a lot of motivation for coming and staying in the field. But I think from now on if someone actually has doubts I would actually point them to this conference and I would ask them to look at the webcast and the video cast because I think this is the best motivation and as someone said earlier, this is really a territory for a young investigator to conquer. And I always tell my students people usually try to go to cancer biology or pathology, I do tell them that this is this condition is probably the greatest intellectual challenge in medicine, and people can really help patients with ME CFS but at the same time really make a career working in the area of ME CFS. That's one the key messages that we all have to work together and we have to help bring new young people into the field. >> I also take a positive note on this and in general. I think one thing this meeting shows is that we're much further along, we don't give ourselves credit for much of what we've have in hand and going. We have clinical trials groups. We don't have to talk about forming them. We already have all these different investigators that are linked together doing clinical trials, they're experienced and they're experienced clinical trials investigators who have the patient population. We have many, many years of experience with these various and sundry instruments we've been talking. So what I'm encouraged about frankly was Dr. Rob and Dr. Slagle's talks that said we can do things quickly, we will help you we want to help you, help is -- lettist help you design your trials, come to this. I have every intention to take advantage of that. And I'm hoping we have pharma partners that will join in that. >> I'm a public health person for 30 years. And so that's sort of the lens that I look through and one of the things you learn in public health is the value of partnerships. And I was pleased -- I really enjoyed all after the talks today. This was a fabulous meeting and I really appreciate the FDA for the work they've put together and all of the participants in the audience and up here. Because I have learned a lot and got a lot of inspiration. One of the things that I heard early this morning was from Dr. Munos and partnerships. That was one of the strategies that he said we needed to do. And so sort of in the strategic way that's how public health works. They reach out and figure out all the different stakeholders and partners that can bring something to the table and so I think that that is a particularly good memg for us to think about, partnerships with academia, about the patient community and all of the different kind of resources that we can use -- get from them and give to them, and from the federal government, from academia, pharma, from foundations, all of those things if we can come together and pool our resources because it can't all come from one place, obviously. Thank you. >> I think the key message that I heard yesterday and today is very familiar one, and I'll give you a hint. You remember when I first moved up here to the DC metro region and wanted to buy home, the voice of my real estate agent was location, location, location. And of course she sit here today me to a very expensive part of town but nonetheless I do love it. But the message that I heard for the past day and a half is measurement, measurement, measurement. And as a psychologist, that is the key to making sure that we have accurate representation of what happens whether it's in a clinical trial, whether it's in clil research, whether it's in any type of things that are in a tissue bank are the things that we're measuring truly representative of what it is that's going on with the patient. And then using those accurate, valid, reliable measures to then devise outcomes and use that to evaluate treatments. And that is the message that I will take home with all of my papers of all the different indications that patients have articulated in the past two days that I was not completely aware of and I will admit readily in the public forum my ignorance of what you go through and I appreciate your honesty in sharing that with me. Thank you. >> So for me as a patient, there's a lot of messages that have come out over these two days. And I'm going to preface this with I am having a bit of brain fog so I'm just going to kind of like run through what I have here. First of all, I want to thank all the patients for attending, because I do know what it is like to try to attend a meeting like this. How much energy you have to put out. One of the things that I have heard the last couple of days and I appreciate it coming from FDA representatives and other clinicians here is that they understand our frustration and our pain. I think they try to, but I would rather ask them to say they would like to understand our frustration and our pain and just for a quick example, on my flight here I set the arrangements up and sent them into whoever the government travel agency was, and it was a flight direct from Reno to Denver 30 minutes I'm on the plane from Denver to here. I got booked on a flight, I had to sit for three hours in the airport in Denver. It's those little things that when a representative says to me I understand your pain and frustration, you're not quite there yet. I know you're trying and we as a patient population will try to educate you the best that we can. I'm very grateful and thankful to Dr. Woodcook and Quitter for arranging this landmark meeting but I am just as grateful to a handful of advocates and patients who pushed to get this meeting today. So like I said, brain fog so I can't name you guys, but thank you for getting this put together and I would ask the patients to applaud those advocates who did this. Thank you. (Applause) >> So what I've heard the last couple days is there's a characterize in the ME CFS community and the government has to take action out of this meeting to address this crisis. We've heard from several key experts that there are biological measures that correlate with patient outcomes that should be used to demonstrate efficacy for ME CFS treatments according to the experts. And those measurements are natural kill are cell numbers and cell function. Dr. Snell exercise. VO2 maximum stress test. Cytokien levels. We herald about viral activation, HHV6. CMV, cognitive function tests similar to those that they're proposing to do for Alzheimer's. We have heard a number of clinicians who have had positive results with treatments with wide are availability and testing and FDA should take concrete measures to help to facilitate clinical trials and clarify a pathway to approvals and we've heard some of the drugs mentioned, heard ampligen, we have heard val treks. Facilitated neets to articulate a superb path to approval for ME CFS. FDA just established a special path for the drug in early stages for Alzheimer's. Recognizing the unmet medical need as there is with this illness. Also recently there was a drug actually approved called sertoro for TB that's receiving a specialty pathway for approval even though it kills five times more patients on drug than on placebo. We have a critical unmet need for 30 years. FDA must make clear to the drug development companies that it will approve drugs for the indication of ME CFS and the only way to correct the 30-year deficit is for FDA to articulate a special path so the drug companies will know FDA is willing to do so. It's been said several times now build it and they will come. Fund it and they will come. When we are talking steps for patients and for patient advocates advocacy community, we need to build off this meeting together we've been very united here. Patients who have spoken have been very articulate. We have made it a point not to just say I have brain fog. People have been very descriptive about what brain fog is to them and that's helped the researchers here and I think is that we have to build off that. We need FDA to push a list of measures that will be accepted as objective outcome measures for efficacy. FDA should recognize and articulate the life-threatening and disabling nature of ME CFS and weighing the benefits of drugs against the risk like it does for MS and cancer and all of those drugs have serious side effects. FDA needs to be willing to approve treatments that work for a subset of ME CFS patients with select criteria so treatments are not withheld because of a heterogenious patient population which has been repeated several times. FDA needs to allow for the smaller clinical trials which they've talked to to be able to demonstrate efficacy with continuing studies after marketing so the path is treatment is not another five to ten years away. I'm 54. I've been doing this since I was 24. I do not want to be sitting up here saying this same thing when I'm 64. If FDA is not able to do -- I'm sorry, we need FDA to publish those criteria and invite the drug companies to set up the clinical trials for ME CFS patients and FDA is not able to do that from the pin put from this meeting and include immune marks FDA should move quickly within the next two months to convene hour expert clinicians with the most experience in ME CFS clinical trials to help create that set of collection criteria and outcome measures. And not least FDA must pursue a fath Ford for amply jen that could reach approval stages in less than five years and it clearly works for a subset of patients. If we could help four percent of our patient population we should do that. We do it with cancer patients. It's done with several other indication and is we should be able to do it for ME CFS. I'd ask CDC and NIH work with FDA and help to study the respond ersz to the drug so they can learn how and for whom it works NIH did this with the MS drug zeno packs and found an implicated a unique cell in that disease. FDA needs to ask NIH to conduct clinical trials in our illness like they did for AZT for AIDS open the door just as they're opening the door for Alzheimer's. I want the Alzheimer's plan. We're willing to loosen the restrictions. I think we need very clear steps coming out of this meeting from the FDA from be able to bring treatment options to patients and their doctors on an emergency timeline. This is a crisis. This is an emergency. This is time for everybody to work together. And try to move in that regard. Thank you. >> I'll be very brief. You but I wanted to take this opportunity to say how impressed I was with the patient testimony that we heard yesterday, particularly in articulating symptoms so very clearly and I think that that's just a huge help in moving forward with outcome measures and guide drug development to know what symptoms to take forward into clinical trials. I found that remarkable and I was, again, utterly impressed with our articulately and clearly that was described how everyone stayed on topic and just really did a tremendous job describing things that despite listening to testimony for several years now I have not heard. >> I would like to echo some comments that have been said but I do believe this is of a great session and I think a session that's allowed many stakeholders to come together and not one person stakeholder or people can solve this. We have to come together. That's very clear. That's one key message that I've gotten out of this. And I think with that guidance can be generated if we come together and be creative about how we might want to do that and the regulatory endpoints might be. It's essential for pharmaceutical companies to understand the endpoints to know what is needed in the clinical trials and what is the threshold to meet substantial evidence. So I think out of the group that's here today and discussions I think we have the right framework to start those discussions and take off from there. I think the other thing that I learned sitting here the last couple days is although it's called CFS and fatigue is a key component of it, it's very complex, and there's many comorbid conditions associated with it. And that also is something that I think as we think about clinical trials something we have to think about. So I think the framework has been established to build off that and I think we have a good spring board to do that. >> Thank you all on the panel members for this excellent discussion on this question. This was very helpful for me and hopefully for all of us here. Before moving on to the next question, I just want to remind us and all of us that we do finish at 5:00 o'clock and that the last 15 minutes of the session is for audience question and answer period and we're going to really keep the 15 minutes for the audience participation here. So with that in mind, I just want to pri up five more questions to go through and we want to give all those questions adequate amount of importance. So we should time ourselves appropriately. We took about 25 minutes or so discussing the first question. I think it's important to get all the points out and with the hope the second and third questions will go rather quickly. So with that, I will pose my second question which is up on the screen. What do you think are the most important factors in facilitating drug development in chronic fatigue syndrome and ME? Perhaps I can start this time from the extreme end and then move down here. Thank you. >> I think there's several questions I would like to pose to this question back, which is what are the criteria by which we need to have in place to register for an indication. We've talked about regulatory path but then what that look like? What are the claims or indicationings that might be associated with it as we've heard anything from signs and symptoms to maintenance and one time I her the word cure. What's that going to look like. The other pieces the clinical heterogeneity we've been demonstrated on here with different speakers. But what is going to be the framework? Is there going to be an academic research organization established, how are we going to be able to implement the trials in such a way that we get the right patients into those to get the endpoints we need? So I think the third pieces eptsd where can we leverage maybe other fatigue instruments we've used and other occasions which maesh may be more secondary but may provide a framework or proof of concept to allow us to know that our drug may work in this syndrome al well. I think that's something else to think about which was hit on in the last session a little bit. >> I'd like to bring out two points here. The first one I think several folks have already commented on which speaks to how much it's resonated with all of us from Dr. Munos's talk about the need for data. In particular it's hard to get to clinical trials with drugs until we have baseline data. And the good news is that we already have a lot of that. But obviously the more you have, the more rich the data set is to build on for drug trials. And in particular, I think longitudinal data is important. What I was so excited about that you folks didn't get to hear is after my panel, we had a brief sidebar conversation and there are a number of different databases already out there that I think give us the opportunity to build on that, and I would just really encourage establishing networks and bringing many of those databases together. They may be different, that's okay. We can look at things in a variety of different perspectives. I'd also like to emphasize the excellence in clinical trial design and the conduct of clinical trials which is so important if you're going to see something, it really has to have a lot of thought into it ahead of time. And when you get to drug trials, I think that's a place where FDA is certainly very willing to help with those designs. >> So for me, I've heard the word a couple sometimes and I think it's willingness, willingness from all the federal health agencies to work together and figure out what the pathway is forward here to to do clinical trials. And I've heard it over the last two days. It certainly appears that the willingness is there again, I don't want to leave this meeting today and have in the back of my mind that we're going to be talking in whatever it is a fee weeks at SIFSAC and not really have some plan in place to be able to move forward. Thank you. >> So from my perspective, and the perspective being NIH is a funder of biomedical research, the basic research on mechanisms and understanding of diseases and illnesses up to clinical trials of a limited nature, from my perspective it's important, I know that we're here to talk about ME CFS and that it's a very debilitating illness and I understand that. But it's very important from a research perspective is to be willing to think outside of ME CFS on the fringe. We're not not going to study this, it's not that we're going to abandon this but be just willing to accept new approaches, new perspectives of individuals who are in other areas of research who have techniques, they have ideas, they have databases, they have other information that they're willing to share with the ME CFS researchers to broaden out how we think about how we do research. And that's very important to consider that from the perspective that basic science research and the limited clinical trials at NIH does fund do those all feed into what pharma will then take on as phase three clinical trials to move forward with drug development. >> Most of what I was thinking about has br been said. I just want to -- I'm a data person and I can't underscore enough the importance of continuing not just gathering data but developing the data infrastructure. And something that I don't know much about but I think something that we can't lose sight of was Susan Vernon's talk today on the drug repurposing. That sounds like something that has some promise in the shorter term to find more useful therapies perhaps drugs that are already approved but then could be either -- should I say off label? Can I say that? Off label or they can -- they could come in for other uses and get it on label. So just to mention that we shouldn't forget the sort of work that we saw demonstrated today in Suzanne's talk. >> I think the most important facilitating factors right now have to do with organizational infrastructure. And if there are avenues to develop the infrastructure that's necessary to really put together a functioning clinical trials group. I can't underscore enough one thing you heard earlier from Dr. Munos is things are cheaper. We have the assessment platforms on these web based plas forms now. They have definitely bring down cost of doing work. And it's very reasonable to think about putting together a series of targeted treatments phase one slash two trials. But -- one slash two probably, to get this on the ground. In terms of sub grouping I think that's other big thing we talk about a lot and -- I think sub grouping can be a strength because it helps us pick out the best population to target that particular approach and not generalize, but go ahead and for instance if we're biologic response modifiers with cascades let's use cytokines to -- there's certainly ways to do that. And we have I think very reasonable strategies to target therapies on targeted groups and do it now. There's really no reason at this point to delay any further. I think we can beat this with our variables to death but we can go to the FDA with our list of variables which I would strongly should be functional rather than symptom, but we have good reliable validated well published compared across each other variables already in addition to these lovely of data sets that we have in hand to further test those variables. So I think that we're ready to go. >> The key word for me that listening to everyone else that spokes on this question and throughout the meeting is collaboration. And I think if you just read the question what do you think are the most important factors in facilitating drug development, I think it's just collaboration and working together and I also want to emphasize the importance of something that Susan mentioned the importance of working together with or researchers and people in similar or related fields. I can just give you a very simple and brief example. So when I was trying to work on a biochemistry marker for chronic pain syndrome, I went and spoke to very famous New York skients at Harvard medical school who's actually now a head of one of the departments, and I mentioned to him that I'm trying to look at the animal model or mouse just because that's what initially a drug company wants or is interested in and he said well, why are you bothering me with this disease, it's a cystitis thing, just put so the bacteria in the bladder of a mouse and then you get your model. I said it's not actually that simple. People with this diseases actually have chronic pain. We assume it's and inflammation but we don't actually know what's going on and it's very heterogeneity disease. And he said that sounds interesting. We actually have a mouse we knocked out the gene and we're working on another disease, but we knock out this gene and these mice have this most weird behavior, they keep picking at their inguinal area to the extent they create wounds but it's such a targeted behavior and it's happening in the pelvic area. Maybe that's what you're looking for. It's an extremely interesting thing chlgts then he showed me the pictures of those mice which actually do head stands. So they have that pain in the area where they cannot use their behind parts and just walk on their front paws. It's the most impressive picture. But I do think that that was a very interesting discussion and I do think that I don't think -- I'm not an expert in this field. I don't think there's a mouse that's representative the the chronic fatigue syndrome phenotype. And I'm not trying to move that discussion in that direction, but all I'm saying is you can actually learning learn a lot from talking to people in other fields and we can learn from each other and probably help the field in a way which you cannot even imagine. >> It's gravity fie to hear people at the talk about how they were learning from patients about the symptoms of CFS and maybe they didn't understand as much previously. I want to thank everyone in the audience as well as those in the internet land hearing about these symptoms. I think a big piece of facilitating drug development is to educate clinicians and researchers about ME CFS. In particular not so much people in this audience or listening to this webcast but doctorate understanding are did a survey back in 2011 presented an abstract showing that 85 percent of physicians think that this disease is either psychiatric or a mixture of medical and psychiatric. It really needs to be emphasized that this is a medical illness. Certainly there are people comorbid psychological or psychiatric disorders and those should be treated but those treatments are not going to really help people recover from this illness. It may help them cope but it's not going to help them recover. I'm always concerned about there old statement in public relations where if you say something enough times it becomes true. Even if it isn't. And we've heard so many times not just here but outside that there are no bother markers for CFS and as people have brought up at this table, there are biomarkers. Not every CFS patient has them. But there are biomarkers that a lot of patients have and we need to work with that with what we have currently. So that's my point. And oh, yeah last point. About working with people outside the field, CFS is not the only disease that has a lot of heterogeneity and not the only disease where if you're conducting a clinical trial there are people with multiple other comorbidities. It's not some neeks sdiez off in its own corner. We can learn lessons from others and apply those to CFS. >> The third question for that we'd like to pose is we heard this afternoon about from panel three about clinical trial design elements and what are the most important ones to ensure drug development programs are created then for CFS and ME. I may want to direct this to Nancy Klimas for her comments. >> Samples size. We're measuring -- we're measuring things that have the ability to change with improvement in a way that you can track and have reliable data and this is really important. The length of the trial itself. To date almost every study that I have seen that even hinted at efficacy didn't see the response for -- in my the fourth month. So designing little ten-week trials or four-week trials may not be enough. Our group is doing something unique and it's pretty cool where we're using exercise challenge to induce a relapse and measuring a lot of things over the course of this exercise challenge. Our intention in phase one is to do that challenge up front, intervene with our targeted drugs, and then do that challenge again and see if it really did change the homeostatic model of the illness. So that's kind of cool and unique is to be able to say let's use computational biology tools which are pretty exciting and see whether or not the intervention works or has a hint of efficacy here but also if it teaches us more about the dynamic of the illness. And I will argue that that would be my perfect phase one study. >> Anybody else would like to comment on that question? >> I would like to bring up one thing that hasn't been very much today that I think is critical that we don't forget. We have very appropriately so spent a great deal of time over the last day and a half talking about measures of efficacy but I'd like us not to forget as we're designing our clinical trials you also have to design in up front measures of safety because that's -- efficacy is only half of the equation. We need to be able to balance risk and benefit and we need to understand clearly what those risks are. Even for drugs that are being repurposed and perhaps have and side effect profile that's well known in other diseases, it may be different in chronic fatigue syndrome and we have to build those into our trials so we're carefully assessing them up front. Otherwise we really can't collect them particularly in a disease like this where there are some heterogenious symptoms it's hard to know what's an adverse events and what's baseline status. >> Thank you for that comment. Anybody else in the panel would like to add anything to what's been already stated on that question, question No. Three? >> I just have two resources that might be interesting to pharmaceutical companies. One is an article by Haywood, a paper on the quality and acceptability of patient reported outcome measures used in ME CFS and its systemic review. Their conclusion was one of the better measures is the SF36 version one but she reviews all these different measures that might be of interest another one is from Australia by Cockshell and that might also be of interest for pharmaceutical companies. >> Thank you very much. If anybody else wants to make any comment on this question? If not, then I'll move on to question No. Four. The question No. Four which is up is what are the most important barriers to conducting research for ME CFS and what can be done to overcome them. The point here is this meeting being patient focused is what can we as a group here can do to overcome the barriers. Listing all the barriers we discussed here quite extensively. With that introduction I'll just throw it open to the panel members here for anybody who would like to take it on. Started with comments and others can add on. >> Just to very clearly put it on the table from the FDA perspective and a regulatory perspective, we don't perceive any barriers. That's not to say that there aren't other barriers and other arenas butregulatory-wise I'm very pleased to say that we've heard this from multiple people. We heard from our panel three members Dr. Rowe outlined is so nicely that we have outcome measures. We also heard it on panel two, Dr. Bateman said any of these symptoms could be used. We heard a number of different measures that could be used. And so we have outcome measures that could be used, we have definitions that could be used and they may not always agree, but that's okay. We can have different clinical trials where tailored to the drug or symptom that you want to measure, you may design your inclusion exclusion criteria slightly differently to precisely define the patient population that's most likely to benefit. And that gets back to the enrichment designs you heard about from Dr. Rowe. So regulatory-wise I think we're ready to go, and FDA is certainly very open to hearing about trials in this area. >> Thank you Dr. Michelle. I think some other speakers earlier said we could not hold a perfect to be an enemy of development and for chronic fatigue syndrome the perfect will come sometime but I think the time is already arrived to enroll patients into trials. At least to make some head way. With that, anybody else from the panel here would like to add their thoughts or words on this question? >> Yes. Can I? >> Please. >> I think that we discussed this on several occasions, we actually had a brief discussion over lunch again with Nancy and Susan and just the table where we were having lunch. But I think one of the barriers that has consistently been pointed out is -- two of them actually. One is the lack of new investigators coming into the field and people that are actually interested in doing additional research on ME CFS and on the other hand we have consistently heard at least at meetings about the limited availability of funds or funding. So some people point out that there's actually not that many investigators. On the other hand, it's been pointed out that there isn't that much availability of funds. In all honesty, I think Susan can speak better to that from the perspective of available requests for applications and other funding resources from the NIH. Buff the one thing that's consistent is that we actually need a concerted effort, probability need all the different clinical centers to come together and probably do a larger mega trial. And it might be interesting to have a discussion, there are obviously different mechanisms under which this can be done at the NIH level which seems to be an interesting way ever doing that. But I just wanted to mention -- so, for example, there is a network that I'm familiar with map network or multidisciplinary approach to pelvic pain which actually has $40 million in funding over five years. And the way this network actually came together was there were requests for applications, money set aside that was dedicated to this network and when the requirement for submitting an application was that every site that was submitting an application must have a clinician recording patients, an expert in the disease, and then loosely defined basic scientific component but mostly something that has to do with biomarker discovery or validation including imaging in that respect. So I think I personally think that the field of ME CFS needs something like that. I think that the field needs a map network. I don't know how that can be done or I don't know the specific mechanism that this can be done. One possibly we just mentioned over lunch is something that I'm vaguely familiar with that the NIH actually has a funding mechanism under a cooperative agreement where interested investigators in clinical trials can work with the NIH over a period of one to two years in putting together a team, designing a clinical trial, bringing people from different sites together working on a specific thing and this is under one. Mechanisms which are called the U34 grant, I think. I'm not an expert in that field. And then following those initial one or two years, when you actually design the trial, put together the team of multiple sites, get the manpower to do that, there's an additional funding period of five years where you actually do the real trial. And I personally think that just listening to what everyone has been saying, that might be a very good way for the field to move forward going with what Nancy was saying the people are there, the sooifts are there. We heard from a lot of experts in the field that they're kree krugt patients. There's interest in testing different medications, maybe that's the way to do it. >> Thank you very much. This is a great comment. Actually what you mentioned here makes very good senses. There are other disease areas where such network has been launched and in the area we're familiar with launch network to study going on for fiech years with actually public funding and sthees are for studying the understanding disease that will lead to future drug development. Those are not necessarily for clinical trials. They're more to n the disease. With that comment I'll ask Dr. Mayor to respond. >> Thank you. >> Sorry, didn't mean to put you on spot. You can say something brief or not. >> I can be really brief. I wrote down an answer to this question and the first thing I wrote down was money. Money is an issue. I'll be honest about that. It's an issue for everyone in the federal government, it's an issue for all of us. And so I cannot make excuses about the money issue. Really what drives the research at NIH is well conceived ideas, good science, and well written proposals and if you write those and you submit proposals and that go through peer review, we can fund it. But we can't fund research without a proposal even if it's a request for applications. I'll give an example. Request for applications as jord Dan stated is a mechanism by which NIH says we're going to set aside x million dollars for this particular activity. Submit your proposals, there are guidelines, et cetera. So let's say $6 million is set aside for a specific activity. Proposals come in, they're reviewed for the science, for technical merit, if there aren't enough proposals to fund at the $6 million mark NIH doesn't spend $6 million on that activity. They can only fund the science that is reviewed by peers to be scientifically acceptable. So an RFA or request for applications that has a set aside to it is helpful, but it's not the be all end all of spending money at NIH. We have to have proposals, they have to be well-conceived, they have to be well-designed, whether they're clinical research or it's a clinical trial and they have to pass the scientific muster and if you submit them and they go for peer review and score well, we can fund them. >>: Thank you for the comment. I think it resonates with the FDA. We approve bug but we have to have an application. I think this is in some ways to the physicians group is for them to -- together for NIH and others to review and hopefully fund it. >> I wasn't advocating for Susan to write a check today. (Laughter). >> Not under sequester. Thank goodness. >> Wait for my furlough day >> Now that we put NIH on the spot, let's ask the question No. 5, please, on the slides. For based on your experience and so forth in the various different agencies and different viewpoints in looking at this conference how can we leverage your experience or your agency's experience in moving drug development forward and we're looking right now at the FDA, for example, Teresa? >> All right. So I think this workshop is a big step for us, and one of the things that's important that we add FDA can do is as you heard, we can't approve a drug if we don't have an application for it. How can we stimulate the field to get more applications. One of the ways that we can do that is by expressing FDA's willingness to work with companies and investigators on deed of trust for CFS. I think that the most important thing we can do is continue to be good ombudsman wherever we are to say CFS, we consider it to be a series disease. That should send a strong message to pharma that this may be a financial tune here. And -money makes the world go round. That's how it works. Thanks I think from a FDA perspective how we can leverage our experience. The other way we can leverage our experience is when people do come in with clinical trials is we can continue to work with sponsors to help them with those trials. We have a vast knowledge base of clinical trial design that while much of it has not been in chronic fatigue syndrome because we haven't seen very many trials in chronic fatigue syndrome, lessons learned from other diseases are directly applicable here. And I was struck by Dr. Rowe's talk and hew he laid out issues with clinical trials. We could take that talk and apply it equally to many other disease that we do have a lot of experience with. I think that we can help you with that. And our division has its doors wide open for applications for chronic fatigue syndrome. >> Can I jump in? Talk to us. Talk to us, don't be afraid to call. If we're talking -- I'm talking from the research perspective. Call me. Email me. If I don't have the answer, I will put you in touch who will provide you with an answer. But if I don't know what you're thinking as a pharma, if I don't know what you're thinking as an advocacy group or what the researchers are thinking, call. Just talk to us and let us help you with the -- I can't write your proposals for you but I can provide technical assistance that will enhance the proposals that come in. And once those proposals come in, they get reviewed, and then we can fund them. >> And how are we going to get the pharmaceutical and biotech companies excited? >> I think today is in in workshop is a good start. I don't know if this was an indication that was on people's radar screen. This is one to do it. The other way I think is understanding what endpoints it's going to take for registration and I understand we've put some forward at this conference which will they be of enough evidence for a benefit risk and also for substantial evidence. I think one thing that struck me is the discussion on the PROs is how can we leverage or PRo consortium and how with we come together to say with FDA and to say what is it we need to measure this with. I think that would really help to understand what that eptd is. And I think I really do believe that today is a -- big stride in that I think the idea that we could actually maybe work together for guidance is going to be another big thing. To help us understand whaens the regulatory hurdle so we can assess what that probability of success is for really the commercial investment made for a lot of these indications. >> Thank you. And Nancy Lee, as far as the HHS goes, >> Right. And I'm going to sort of talk out turn and speak for one of the agencies that I don't directly represent because I'm not in the agency but I'm going to talk a little bit about what I know which is epidemiology. We need to have the infrastructure and the work that CDC is doing and is really about developing that. So as a concrete example. Beth showed some wonderful slides that showed the distribution of the various symptomatology. So now we have -- that's not a representative sample of all of the people with CFS we know but it's a start. And if you can use that information and you pick whichever of those symptoms you're going to kind of think about, then when you do your sample size calculations, you're gonna have a better idea of really how many people you need -- how many patients you need to have your study. I was saying a while ago I don't know how -- they mutt not having a statistician on some of those early trielgs where they had 15 people because there's no way. I don't know how they even thought they would get anything out of that. Knowing that these are sort of the prevalence of the symptoms and the likelihood of response. So having the data to really help us figure out the best way to decide how to move on the trials is important and CDC is doing important stuff on that and I know they're planning on get some in the next phase of the study, they're going to be some blood work, et cetera. And so that's what I want to say. >> Thanks. >> Excuse me, Dr. -- may I? >> Okay. We're going to put you next here. Go ahead. >> Thank you. Wanted to respond to what doctor mayor was saying earlier about submitting grants and getting them funded. And I'm going to kind of just take this and throw this to Dr. Klimas because I think yesterday you were talking about how many applications that you've put in and what the response is. So I would ask you to reiterate that. >> Gee, thanks. I don't know if this makes me look bright or dull. But, yeah, we have about an eight to one ratio for funding. We write eight and we get one funded. I think I've only once in my life been funded the first time I submitted something. It's always on the second can't go three times anymore, so the second round. But since I got the microphone I'm going to take the academia perspective that's up there and and say that I think the best way for drug development to leap forward right now, I'm going to be bold and crazy and say right out front, we needs philanthropic support and cut two years of a. If I have to go in for phase one, I have an excellent study submitted three times to three different agencies, I've had it turned down three times. I should have had it in play two years ago. Coming right out of our computational modeling work and we could say it wasn't well written and people didn't understand it or whatever, there's goods reasons why it might have been accepted but in the end I could have already done the phase one study while I waited around to try to find the funds to do that study and it wouldn't have cost that much. So it's very frustrating and then you all the want us to be so much quicker at getting you your through phase three times two get the label on, clinical trials work so that you can have your drugs. I'm struggling to give you your phase one slash two work that would lead to the phase two, two slash three, three works that would eventually end up with a label on the drinking and there are a lot of different opportunities of things to study, a lot. It's not that we don't have good ideas and good science to go behind those ideas. What we don't have is your time to waste. If there's something that we could do that would just knock two years off the time clock it would be starting from foundation or philanthropic support to do that work. We have a translational medicine unit and it's the number one funding. Let's get it going. And it's very exciting. And it's definitely what we should be doing right now. We'll still knock at the right doors, all the other doors. I've got nine grants on the board right now that we're going to be submitting between now and next Christmas. We're all working very hard on your behalf, but maybe one of them will get funded. That would be cool. >> Thank you again for the panel for this discussion. >> I have three points. Point number one, the survey that did I earlier I wasn't able to present the results so if anyone wants a page and it has to do with the epidemiology of CFS you're welcome to email me at lilyxchua Gmail.com. So that's number one. Point new two as a bored I want to invite anyone interested in learning more about CFS to join our organization or to join or meeting in San Francisco next March. We publish a journal and a quarterly newsletter. We're open to accept different types of articles and on the other side we can help disseminate information to our clinician and research members about, for example, funding or collaborative opportunities. That email address senior the forgs is www.iacfsme.org. As a patient, I want pharmaceutical companies and other people to understand patient groups are enthusiastic and willing to work with anyone have advance progress on this illness. If you need help with recruiting patients to a trial, there are many organizations that work together now patient organizations and one of them is the national advocacy alliance for ME which consists of many of state groups across the country. Their web page is www.naame.org. Thank you. >> Thank you very much and in about two already three minutes we'll be going to the next phase which is taking questions from the audience. Before that her is question six and very briefly ask the panel members to comment on that. The highlight of the question is next steps forward. With that I'll turn it over to anybody who would like to speak. Please. >> It's the obvious thing that we need to have a small meeting with the experts in the FDA to talk about these outcome variables and really nail some outcome vertebral stuff down. That would be very, very helpful and I would volunteer to come back to DC and do that with anyone else that wants to do that. (Applause) >> Anybody else from the panel here wants to comment on this? >> I think I've kind of already stated this but I do think that going into a guidance or some regulatory precedent of what we need to do to devise trials is going to be essential to move this forward. So that we can understand kind of what that is over all and what's needed for that. >> Please. >> Thank you. So as my colleague from Lily so nicely put guidance for industry is definitely I think an important next step. It's something that we here at FDA are already thinking about. I suspect that we'll be able to gather a lot of the information that we have heard of here at this meeting and begin to formulate that and we'll need to continue to gather some additional information as we move forward and there are a varlt of sources to look for that, including information that the CDC and the NIH are currently developing. So I think we have a lot of resources in that area and we hope to be working on that definitely over the next months to year on or is, takes a long time for a guidance from FDA just the logistics of getting it through the system. So don't look for it next month, but it's definitely something that we are very well aware of and people sitting next to me can see that I already have it circled on my paper. So thank you for that. >> Thank you. Anybody else would like to make a brief comment? >> I just wanted to make two comments that are brief actually. I volunteer to come back as well and I I don't have to be paid for that. If the FDA actually has a smaller meeting with just art calculating the specific endpoints. Another thing I wanted to bring is that during one of the conference calls we is that might be helpful if the peer reviewed paper comes out of this discussion just to inform medical community about the progress it has been made over the past two days so that actually the community at large has some better idea about the field moving forward so it would be a good idea if we can work on this together as a group or with -- if other people are interested in doing that. (Applause) >> Adding to Jordan's point this is publishing in a peer reviewed journal would also be a slentd way to recruit new investigators and people from other fields that might not have realized that their fields overlap with CFS >> I would like to move on to the next set which is questions from the audience but before I do that I wanted to make sure if anybody else from the panel here wants to make any comment. No? Okay. The audience here are listening carefully writing very important interesting questions. Which want to give these questions time and discussions. So with that I'll put up three questions together and try to answer them myself or pass it to the panel. First is other clinical FDA trials approved for CFS, why exactly for ampligen was not approved and does FDA have working group to write industry guidance. The guidance question Dr. Michelle sort of answered and for the other questions, for the for the drug there was an open public meeting and the materials are on the website. Why that was approved just as a point of in the process the FDA takes a decision writes a letter to the company and that is confidential for the companies that want to hold onto it. If they wish to make it public they can make it public. We can from the FDA cannot. So I think the materials are on web and one can review that. And are there other clinical FDA trials proved for chronic fatigue syndrome and as Dr. Michelle earlier mentioned, there are but there are not too many. With that, I'll turn to Dr. Michelle. Do you want to add anything on these questions? The questions were do we have a working group and a guidance writing activity. >> I think you covered it beautifully. Thank you. >> Okay. >> I wanted to ask, he said there were other clinical trials. Can you tell me what those are? Specific to chronic fatigue syndrome -->>: Dr. Michelle is going to the microphone to respond to that >> Right. So this was in the presentation and I'd encourage to you take a look at those slides up on web at the SIFSAC website and Nancy Lee probably noesz the url off the top of her head >> Just Google SIFSAC. >> That's how I get there. And it outlines exactly the number of trials which isn't a lot. And the the number of active INDs which is a handful. >> Okay. And Jody, the question comes in here, it says can you comment on how investing in repurposing of currently available drugs for CFS, a financials gi worthwhile investment for a pharma company? >> , first of all, I think there's some fundamental principal pelgs of the pharmaceutical company uses. We continue to look for opportunities for that drug to be used one we establish it for its initial indication and that helps many different patient. We're looking nor what we call line extensions basically to add onto that drug. I think the atoms that factor into that are no different than the initial indication which is what's your regulatory pathway, what's your endpoints and what's your likelihood of success to be successful in that indication based on what you've demonstrated in other areas. You build with your safety buff the safety may be different in CFS so how do you establish the safety and efficacy in in a new indication. You build a commercial case as well as a risk benefit case and build that forward to say is that an indication that's worthy of investing, is it going to serve patients and is this particular drug would proof of evidence it's going to work in this population which is another one of the key attributes. >> Thank you. Questions come in here which I just love here because I don't work for the NIH anymore and I can actually ask this question. It says is anybody lobbying congress to set aside money for CFS research? Lily can -- there's not too many people up here that can answer that. Go ahead. >> Well, I've been trying to lobby congress to set aside money for CFS research. And it's not easy. I think the legislative aids I've talked to and congress people are interested but my problem is I don't have the energy to do it, to follow up. And that's my biggest issue. I've tried getting more people interested in doing this but it's hard just because I have really very little energy to coordinate so I'm hoping that people will take this up or work together. >> So when you lobby congress, the most obvious pot of money that woo then go into is a DOD pot. Zoo if its association in others successfully lobbied congress and put aside some money into that pot last year. So however as a -- iks tell you that the DO did is a wonderful place. The office of congressional research has a very professional outstanding group of people trying to push those protocols forward. They take is very seriously and do an excellent job. I'm in a Gulf War illness con some shum that was just award add $4 million a year consortium and we were successful and actually the second consortium I put in was also successful. They funded two last year. This year they have $25 million for Gulf War illness. Last year it was $19 million. The VA has $19 million this year for Gulf War illness. Does this sound cool to you thinking wow if my field had -- and it's really true. When you think about that they're spending maybe $40 million a year on Gulf War illness which affects a quarter of a million veterans who went to the first Gulf War. It's a verbal illness. Feels just like chronic fatigue syndrome. And is very different in a biologic level and veryoverlapping in other areas so it's a fascinating illness to be studying side by side with chronic fatigue syndrome and had gives us some very sophisticated and well funded ways to better approach these illnesses. >> My he person approach has been to put chronic fatigue syndrome as a compare thor group for many years >> Because I have heard about this alternative source of federal funding and my understanding is their process is very, very inclusive of patients. Maybe more so -->>: At the review level. >> Very, very inclusive of patients. And they're very -- they get their response -- they make their decisions based on the input of the advocates that get to congress and convince them. >> I want to echo what Lily said. I've spent quite a bit of time going up on the hill and it's very difficult because you get a meeting set up at one place and you got 130 minutes later in another building, takes a lost time and energy buff it's certainly worth doing and I would ask patients watching and sitting in the audience, you don't necessarily have to go to the hill to lobby. You can write your senators or congressmen, and they will respond. I have several letters. >> Local office as well >> Yeah. Thank you. >> Thank you. I think we have discussed this question. I have two more questions to briefly go through and these are somewhat long questions. The first one is direct to Dr. Michelle and this is on safety evaluation specifically serious adverse events. The question is what doctor Michelle to respond to this question. Serious adverse events all hospital admissions are considered SAEs since 2010 although not drug related nor serious. This complicates safety evaluations of an IND with non-drug related adverse events. Can FDA review this and sort of clarify. I think the question here is hospital admission is being considered serious drug related versus not drug related. >> Right so the definition of a serious adverse events is well codified in the guidance documents and it's also in the code of federal regulations so we don't really have much leeway to change that, but what we do do in clinical trials is that's the beautiful of a comparer group, you have your drug treated patients and patients not being treated with that drug and you look at the serious adverse events that occurred in both groups and you compare them. That's also why it's so critically important in a heterogenious disease like this solve a large enough database. If you're talking about one or two events it's hard to know what think really mean but if you have a large enough database then you can see adverse events that occur more frequently your treatment group. >> Last question for the day, the question is on biomarkers. It says that we have biomarkers. Why does FDA continue to insist we have none? What needs to be done to validate immune markers as biomarkers? Let me try to respond to this as one way of looking at it is it really necessary to have a biomarker to have a drug approved. The answer is no. If one shows benefit on a clinically meaningful endpoint to a patient one does not need a biomarker. And how does one validate biomarker it becomes very complicated. Biomarker -- physical functions is another which we have heard earlier, it gets validated and then becomes at some point -- reflection of the actual clinically meaningful endpoint. And then at some point even it may be considered to be real outcome as one mentioned about cholesterol. So the point here that I would respond that I think for chronic fatigue syndrome there is no up front need for drug approval process for an IND study and drug evaluation and research to develop a biomarker. >> As we enter into the era of clinical trials now is our chance to test our biomarkers but as you heard before, the primary outcome variable might be something like function. And your secondary outcome variable might be a series of biomarkers you'd like to test against that primary to see if it's going to be a decent surrogate or not. In early trials you can throw in some tra variables that are bioplaeshgs and then you're collecting this validation data set so when you third that HIV work I was a part of that very first AZT trial. I was there. And the second and third. Death was the primary and then we had CD four count initially as secondary and eventually a viral node as secondary which turned into our primary and we could approve drugs faster because we were able to prove something actually did predict death. We need something that will predict function or some other variable that's very important but I would argue function and chronic fatigue syndrome and yes, I do think we have the data to support that there are biomarkers in our hands right now that predict function. In fact we've been publishing them and present being them and you've heard them. But they need now to be attached into clinical trials as secondary outcome variables and then I think we could be make a compelling case for drug development for drug trials which is a little different than what your will your insurance company pay for but for drug trials that these things are actually useful and could become surrogates >> Anybody else wish to comment? >> Well she's been helping me with this, but fibromyalgia has drugs approved for it and there are no biomarkers and it was approved basically on the functional -- clinical outcome. So that's a condition that's has a lot of overlap with ME CFS >> I'd like to comment I think biomarkers are really important, and it's a little different but I think they're very important for inclusion criteria and selecting subgroups. Because even as Dr. Unger pointed out in their studies, you can have clienl phenotypes but it may be difficult as a way to distinguish different groups. Although in my opinion I this the the orthostatic intolerance subgroup might be a very interesting one to explore. >> Thank you very much. >> I think biomarkers are very important and I would come back to the regulatory endpoint as sometimes more informative. I think boeshg markers have their own set of hurdles to get in but I think both need to be studied and this based on where we're at with this condition. But the regulatory endpoints of what is going to be meaningful in a label is kind of what I've taken out of this is going to be the utmost importance to move a drug through most expeditiously for the patients. >> Anybody else have any comments on this question or any other questions? >> I wanted -- AZT was approved with a requirement that you had to have a CD4 countless than 200 to be allowed to have the drug and CD4 count had not been approved as a biomarker for anything. So we were using a research only biomarker for about a year before they got the biomarker will be able to on to the things that we had he had to do in order to get to the drug. Sometimes you move on forward. >> Thank you very much for this comment and again thanks to the panel for a very interesting exciting discussion here (applause). Thanks. Thanks to the audience staying engaged putting together a lot of interesting questions. We really appreciate it. And before I pass it on one small announcement I have to make is Mary [inaudible] has been asked to me somebody at the desk which is outside the desk here. >> Again I really want to thank this final panel. I think you did a really wonderful job of pulling together a lot of the points that have been made over the past two days really including going back to yesterday with the real very focused patient discussions and then bridging us to clinical research and the bigger picture of developing products that can be helpful in this serious disease. It was really a great job. (Applause) I also want to thank you all of you in the audience who have persisted, probably need to stretch right about now. This meeting has really -- I would say far -- it has met and exceeded our expectations, and a lot of the reason for that is is because you have been here. You have engaged, you've asked really good questions. And you have rolled up your sleeves and shown up. I always tell my kids, showing up is half a life. And you've shown up. And for many of you, you've shown up in the face of great difficulty. And I personally and I know all of us at FDA who have been working on this meeting appreciate that greatly. We have started on an important path together. There has been and I'm really excited to see all -- it was very excited to see how much work and energy and passion already exists in this field by clinicians, by investigators, most of whom are working a lot on their own and don't have a lot of opportunities to come together. And if I heard one thing in this meeting it is the importance of collaboration. And I would urge everyone who has an interest in this field to take that message to heart and to take another message to heart. What will make a difference is personal leadership, personal leadership. Not the leadership of this group, that group, the government, DOD, whoever it might be. But the sense of personal leadership among people who have a passion for this. Who are willing to roll up their sleeves and say what can I do? Who can I collaborate with so that we can get something productive done? Who can I listen to who might approach this from a way that is a little different than I've been thinking about this? Who maybe I've had some conflicts with in past about the best way, how can we come together and find our common ground and really make some progress? I can tell you from the comments that I had heard on this last panel that it seems to me that most people here at this table are expressing a willingness to do that. And I want to challenge you all to bring that to fruition and I want to challenge those of you in the audience to work with us all collectively to make that happen. Two or things. First, this meeting in its entirety yesterday and today, has been videotaped, people have been watching it on their computers at home and those of you who are with us remotely I want to thank you for sticking it out. For those of you who want a refresher, the whole thing is going to be available on the FDA internet site so that you can watch sections that you want to review again, and you'll be able to watch them with the slides up close because the slides are going to be posted on the FDA's website as well and we'll make sure that they're easy to find. I also want to thank the people in the room who have not been participating in asking questions and who have not been sitting up here at the table. So I particularly want to see the people who are in the back who have been really doing a lot of the leg work. In particular I'd like to see Mary Gross over in the corner, Mary Gross has been (applause) our absolute task master inside the FDA making us come to meetings, making us send the slides, making these people send the slides and keeping us on track and Randy Clark, where is Randy? She's hiding. Stand up, Randy. One of the most positive forces we know. Helps gets things done and always with a smile. She has great ideas. We don't that you were going to contribute that Randy. She just overall wonderful. And these are the kind of people at FDA and there are many many others people in our -- people like Richard Klein and David Banks and our patient outreach groups, our policy folks have been very involved but you haven't seen them. And and we we put this kind of meeting together it's people from across our center who really do come together and put their heads together and take their work very seriously. To them, this is public health. And that's why they come to work every day. And it's because of things like this that they wake up in the morning and say I am going to make a difference. And so we hope that we can all continue collectively to make a difference. I want to thank you again for your inquisitiveness, commitment, your willingness to ask questions, your willingness to challenge each other and wish you a safe travels and a wonderful rejuvenating weekend. Thank you very much. (Applause)Event is not active