Set6ans
advertisement

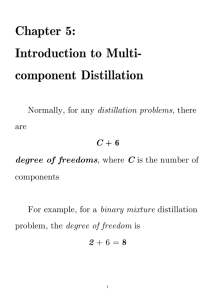
CHE425: Problem set #6 1) Run program ABSORP. You need to copy the folder CHE425 into the H: drive or your flash drive. Open the folder CHE425 and double click on DOSBox.exe. When the prompt “C:\>” appears, type ABSORP and press “ENTER” to run the program. Copy and report the score and performance number from the program. Type “e” or “exit” and press “ENTER” to exit the DOSBox program. 2) 1We have 10 kmol/h of a saturated liquid feed that is 40 mol % benzene and 60 mol % toluene. We desire a distillate composition that is 0.992 mole fraction benzene and a bottoms that is 0.986 mole fraction toluene. CMO is valid. Assume a constant relative volatility with BT = 2.4. Reflux is returned as a saturated liquid. The column has a partial reboiler and a total condenser. (a) Use the Fenske equation to determine Nmin. (b) Use the Underwood equation to find (L/D)min (c) For L/D = 1.1(L/D)min, use the previous results and the Gilliland correlation to estimate the total number of stages and the optimum feed stage location. Ans: Nm = 10.37, Rmin = 1.7488, N = 24.6 3) 2We have an existing column that acts as 30 equilibrium contacts. We are separating a multicomponent mixture where LK = 1.1 and LK = 1.0. A recovery fraction of 98% is required for the light key in the distillate. Find the recovery fraction of the heavy key in the bottoms. Feed is a saturated liquid that contains zLNK = 0.1, zLK = 0.4, zHK = 0.3, zHNK = 0.2. LNK = 1.25 and HNK = 0.75. Operation is at total reflux. Ans: FR of HK = 0.263 4) The following feed of 100 mol/h at the boiling point and 60 psia pressure is fed to a fractionating tower: n-butane (xa = 0.40), n-pentane (xb = 0.25), n-hexane (xc = 0.20), and nheptane (xd = 0.15). This feed is distilled so that 95% of the n-pentane is recovered in the distillate and 95% of the n-hexane in the bottoms. Calculate the following. (a) Moles per hour and composition of distillate and bottoms. (b) Top and bottom temperature of tower. (c) Minimum stages for total reflux and distribution of other components (trace components) in the distillate and bottoms, i.e, moles and mole fractions. (d) (L/D)min using the Underwood equations. (e) For L/D = 1.3(L/D)min, use the previous results and the Gilliland correlation to estimate the total number of stages and the optimum feed stage location. Table 4 Equilibrium K values for light hydrocarbon systems ============================================================= (1) ln K = A/T2 + B C ln(P) , where P is in psia, T is in oR 1 2 J. D. Seader and E. J. Henley, Separation Process Principles, Wiley, 1998 J. D. Seader and E. J. Henley, Separation Process Principles, Wiley, 1998 1 Compound A B C Form ============================================================= n-Butane 1280557 7.94986 .96455 (1) n-Pentane 1524891 7.33129 .89143 (1) n-Hexane 1778901 6.96783 .84634 (1) n-Heptane 2013803 6.52914 .79543 (1) Ans: (a) Moles per hour and composition of distillate and bottoms. D = 64.75 mol/h, B = 35.25 mol/h (b) Top and bottom temperature of tower. Dew point temperature, T(R) = 607.22, T(C) = 64.19 Bubble point temperature, T(R) = 733.02, T(C) = 134.08 (c) Minimum stages for total reflux and distribution of other components (trace components) in the distillate and bottoms, i.e, moles and mole fractions. Nmin = 7.74 Table C Product distribution Distillate xd Bottoms xb -3 nC4 40 0.6178 2.38×10 6.75×10-5 nC5, light key 23.75 0.3668 1.25 0.0355 nC6, heavy key 1 0.0154 19 0.5390 nC7 2.0788×10-3 3.21×10-5 15 0.4255 64.75 1.0000 35.25 1.0000 (d) (L/D)min using the Underwood equations. Rmin = 0.5658 (e) For L/D = 1.3(L/D)min, use the previous results and the Gilliland correlation to estimate the total number of stages and the optimum feed stage location. Ne + Ns = 18.7, Ne = 10.2, the feed tray is 10 trays from the top. 5. Run the ChemSep program and produce Table 5 in the example: Distillation Calculation using ChemSep Software. Copy the ChemSepL folder from the Distribution folder to your H: drive. Open the ChemSepL folder and double click on cs.bat to start the program. Note: This program only works properly in your H: drive. If you want this program on your flash drive you need to download ChemSep-LITE from the website: http://www.chemsep.org/program/index.html and install it on your flash drive. Example: Distillation Calculation using ChemSep Software “ChemSep is a software system for simulation of distillation, absorption, and extraction operations. ChemSep integrates flash calculations, the classic equilibrium stage column model and a nonequilibrium or rate-based column model in one easy to use program”.1 ChemSep-LITE is available for FREE. It only includes the equilibrium column model and is limited to 10 components and 150 stages. 1 http://www.chemsep.org/program/index.html 2 Double click on the cs.bat and the following screen will appear You can click on the menu: Components, Operation, Properties, Feeds, and Specifications on the left column to specify your operation and conditions. When all the necessary specifications are entered into the program, the menu will have a green check next to it. Let consider the following example: 3 A butane-pentane splitter is to operate at 8.3 bar with the following feed composition Saturated liquid feed (kmol/s) Propane, C3 .05 Isobutane, iC4 .15 Normal butane, nC4, light key .25 Isopentane, iC5, heavy key .20 Normal pentane nC5 .35 For a specification of a reflux ratio of 2.5 and split between two components in the bottom iso-pentane/n-pentane = 0.45, determine the compositions of the product streams. You can enter the data given in the example and the following information into the program: Operation Equilibrium column Simple distillation Total condenser Partial reboiler Number of stages: 14 Feed stage: 6 Specifications Constant pressure Column heat loss: 0 Default stage efficiency: 1 Thermodynamics Properties K-value: EOS Equation of state: Soave-RK Enthalpy: Soave-RK Leave blank for Thermodynamic Model Parameters Column Specifications Split between two components iso-pentane/n-pentane = 0.45 Feeds State: Pressure & Vapor fraction You will need to explore this program to be familiar with the menu and sub-menu. You should read the ChemSep Help and ChemSep Book to know more about the program and its functions. After you enter all the necessary information into the program you will arrive at the following screen: 4 Choose Quick Solve in the menu and you will get the following results: Table 5 Result Stream Stage Pressure (N/m2) Vapour fraction (-) Temperature (K) Enthalpy (J/kmol) Entropy (J/kmol/K) Feed1 6 Top 1 830000 0.000000 355.242 -1.428E+07 -41313.7 Bottom 14 830000 830000 0.000000 0.000000 338.339 384.767 -1.548E+07 -1.032E+07 -48735.5 -33575.1 Mole flows (kmol/s) Propane 0.0500000 0.0499994 5.5389E-07 Isobutane 0.150000 0.149712 2.8757E-04 N-butane 0.250000 0.246904 0.00309628 Isopentane 0.200000 0.0630532 0.136947 N-pentane 0.350000 0.0456737 0.304326 Total molar flow Mole fractions (-) Propane Isobutane 1.00000 0.555342 0.444658 0.0500000 0.0900335 1.2456E-06 0.150000 0.269586 6.4674E-04 5 N-butane Isopentane N-pentane 0.250000 0.444597 0.00696328 0.200000 0.113539 0.307983 0.350000 0.0822443 0.684406 6) Use ChemSep to simulate a distillation column with 100 mol/h of feed at the boiling point and 410 psia pressure and the following composition: n-butane (xa = 0.40), n-pentane (xb = 0.25), n-hexane (xc = 0.20), and n-heptane (xd = 0.15). You can use the following specification: Operation Equilibrium column Simple distillation Total condenser Partial reboiler Number of stages: 19 Feed stage: 10 Specifications Constant pressure Column heat loss: 0 Default stage efficiency: 1 Thermodynamics Properties Feeds K-value: DECHEMA State: Pressure & Vapor Activity Coefficient: Regular fraction solution Vapor pressure: Antoine Enthalpy: Soave-RK Column Specifications Reflux ratio: 0.75 Split between two components N-pentane/N-hexane = 0.004 Report the following. (a) Moles per hour and composition of distillate and bottoms. (b) Top and bottom temperature of tower. Click on the FUG Result and report the following (c) Minimum stages for total reflux (d) Minimum reflux ratio. (e) The total number of stages and the optimum feed stage location. Stream Stage Pressure (N/m2) Vapour fraction (-) Temperature (K) Enthalpy (J/kmol) Entropy (J/kmol/K) Mole flows (kmol/s) N-butane N-pentane N-hexane N-heptane Feed1 Top 10 1 410000 0.000000 341.454 -6.363E+06 -21979.0 Bottom 19 410000 410000 0.000000 0.000000 327.571 406.294 -7.984E+06 5.9245E+06 -34525.7 10996.5 40.0000 40.0000 1.0500E-05 25.0000 24.9237 0.0763508 20.0000 0.912304 19.0877 15.0000 1.8929E-04 14.9998 6 Total molar flow Mole fractions (-) N-butane N-pentane N-hexane N-heptane 100.000 65.8361 34.1639 0.400000 0.607569 3.0736E-07 0.250000 0.378571 0.00223484 0.200000 0.0138572 0.558710 0.150000 2.8751E-06 0.439055 7