Chemistry PPT Flashcards Unit 3

Chem PPT Flashcards, Unit 3
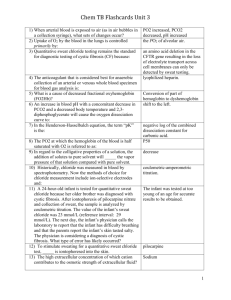
What is the function of electrolytes? Run maintenance of water homeostasis, maintenance in acid-base balance and muscle functions as well as serves as cofactors for enzymes.
What is the major cation of extracellular fluid? Sodium (Na
+ )
The osmolality. What does Sodium determine in the extracellular fluid?
When is sodium excreted in the urine?
What happens when serum levels are below 110 mmol/L?
Sodium specimens consist of…?
And should be stored at…?
When serum sodium exceeds 110-130 mmol/L
All the sodium in the glomerular filtrate is virtually reabsorbed in the proximal and distal tubules, a process that is influenced by aldosterone.
Serum, plasma and urine specimens and may be stored at 4°C or may be frozen. Lipemic samples need to be ultracentrifuged.
What are the methods of measuring sodium?
What is the major intercellular cation?
How are high concentrations of potassium maintained?
Does potassium exhibit a renal threshold?
Atomic Absorption Spectrophotometry (AAS),
Sodium Ion selective electrode (ISE), and
Spectrophotometry.
Potassium (K
+
)
Na
+
K
+
adenosine triphosphate (ATP) pump which is fueled by oxidative energy and continually transports K + into the cell against a concentration gradient.
No, however it is excreted into the urine even in K-depleted states.
What are some of the functions of potassium? Regulation of neuromuscular excitability
(both hypo- and hyperkalemia can cause muscle weakness) and contraction of the heat and cardiac rhythm (decrease K
+
increases
How does potassium affect acid-base status? cardiac excitability and often leads to arrhythmia. High K + slows the heart rate).
In hypokalemic states, sodium and H
+
ions move into the cell to replace K
+
. The H
+ concentration is therefore decreased in the
ECF=alkalemia (reverse is true of hyperkalemia).
What should the concentration of K
+
be?
How does the release of K
+
affect values?
In plasma and whole blood the concentration is 0.1-0.7 mmol/L lower than those in serum.
As few as 0.5% K
+
of RBCs will increase K
+ values by 0.5 mmol/L. An increase of K
+ of
0.6% has been estimated for every 10 mg/L of plasma hemoglobin (Hb) caused by hemolysis.
1
Chem PPT Flashcards, Unit 3
What causes glycolysis to be inhibited and the energy-dependent Na + , K + -ATPase will not maintain the Na
+
/K
+
gradient?
What does a K + leakage from erythrocytes and other cells cause?
What causes falsely decreased K
+
value?
When a whole blood specimen is maintained at 4°C versus 25°C before separation.
It causes an increase in plasma K + .
What are reliable determinations recommended for K
+
?
When an un-separated sample is stored at
37°C because glycolysis occurs and K + shifts intracellularly. Leukocytosis will initially cause falsely decreased K
+
concentration at room temp.
Collect blood with heparin, maintain near
25°C and separate the plasma within minutes by high-speed centrifugation without cooling.
What can falsely increase K
+ concentration?
What are methods for the determination of sodium and potassium?
What is the major anion of the extracellular fluid?
What is the function of chloride?
Skeletal muscle activity as a result of repeated clenching of fist and application of the tourniquet.
Ion selective electrodes (ISE) and spectrophotometric methods.
Chloride (CI
-
)
Where is chloride absorbed?
What specimens contain chloride?
How is chloride affected by hemolysis, change in posture or stasis, and tourniquet use?
Methods for chloride determination?
Maintains the water distribution, osmotic pressure, and anion-cation balance in the ECF.
In the intestinal tract and is excreted by kidneys.
Serum, plasma, urine and sweat.
It is not affected.
Coulometric-amperometric titration of chloride (cotlove chloridometer technique) and ion selective electrode methods.
Measurement of sweat chloride (sweat testing)? Cystic fibrosis, the most common lethal genetic disorder of Caucasian population characterized by increased sweat chloride concentration.
Sweat testing and newborn screening… Are performed in conjunction, with a positive screening test are referred to as a quantitative
What phases are done for sweat testing? sweat chloride test.
Sweat stimulation by pilocarpine electrophoresis, collection of sweat, qualitative or quantitative analysis of sweat, sodium or conductivity.
What is an abnormal infant sweat testing result? ≥60 mmol/L = indicative of CF
Bicarbonate is another name for: Total carbon dioxide
True or False: Plasma or serum can be used to measure bicarb?
True
2
Chem PPT Flashcards, Unit 3
True or False: Sample must be centrifuged in an open tube?
True or False: Ambient air contains far more
CO
2
than plasma?
If CO
2
is allowed to escape from the sample into air, ___to___ mmol/L will be lost per hour.
Two ways of measuring total CO
2 are acidification and ____
Acidification is a(n) ___ electrode based method?
Define osmometry:
False, be must be unopened
False
4 - 5
Alkalinization
Indirect
Define osmotic pressure:
Define osmosis:
Name 4 colligative properties of solutions:
Colligative properties of solutions are all directly related to:
The term osmol al ity expresses:
The term osmol ar ity expresses:
Plasma and urine osmolality is useful in the assessment of:
The 4 major osmotic substances in normal plasma are:
The Henderson-Hasselbalch equation defines pH as:
The Henderson-Hasselbalch equation is widely used to calculate the ___ point of proteins.
Total O
2
content (cdO
2
) is: a technique for measuring the concentration of solute particles that contribute to the osmotic pressure of a solution. the pressure required to stop osmosis through a semipermeable membrane between a solution and pure solvent. process by which molecules of a solvent tend to pass through a semipermeable membrane from a less concentrated solution into a more concentrated one.
1. Increased osmotic pressure
2. Lowered vapor pressure
3. Increased boiling point
4. Decreased freezing point total number of solute particles per mass of solvent. concentrations relative to mass of the solvent. concentrations per volume of solution.
Electrolyte and acid-base disorders
Na + / Cl / glucose / urea
The negative log of the H isoelectric
+
activity
Oxyhemoglobin (O
Define the following terms: pO
2
= pCO
2
= ctCO
2
=
HCO
3
= cdCO
2
=
2
Hb) is defined as: the sum of the concentrations of hemoglobin-bound
O2 (oxyhemoglobin) and of dissolved O
2
(cdO
2
). erythrocyte hemoglobin with O2 reversibly bound to Fe2+ of its heme group. pO
2
=partial oxygen pressure. pCO
2= partial carbon dioxide pressure. ctCO
2
=total concentration of carbon dioxide.
HCO
3
=bicarbonate. cdCO
2
=concentration of dissolved carbon dioxide.
3
Chem PPT Flashcards, Unit 3
What specimen is used for blood gas analysis? Whole blood.
What is the only clinical reason for an arterial PO
2
value draw?
PO
2
is generally 60 mm Hg ___ in venous blood.
Lower
Higher PCO
2
is 2-8 mm Hg ___ in venous blood.
Arterial and venous specimens for blood gas analysis are best collected:
Lyophilized heparin is preferred to liquid heparin because: anaerobically with lyophilized heparin using glass syringes liquid heparin dilutes the sample, and the effect is greatest when the syringe is not completely filled
Increase in pO
2
, increase in pH, decrease in pCO
2 greater
3 effects of exposing blood gas samples to the air:
The pCO
2
in blood is much ___ than the pCO
2 in the air.
On exposure of blood to the air, the total CO2 and the pCO2 both ___
On exposure of blood to the air pO
2
__
In blood gas samples, clots are ___
In blood gas samples, air bubbles cause
(increase or decrease) in total CO
2
, pCO
2
, pH, pO
2
.
What are the reasons for the following changes in pCO2, pH and pO2 in a sealed specimen left at room temperature for 2 hours:
Arterialized capillary blood is an acceptable alternative to arterial blood but it has to be:
The first drop is discarded and the subsequent free forming drops should be taken up in a: decrease increases unacceptable
Decrease, decrease, increase, increase.
Increase in pCO capillary collection tube containing lyophilized heparin
30
2
as a result of continued metabolism, decrease in pH due to increased production of carbonic acid and lactic acid during glycolysis, decrease in pO
2
because O
2 is consumed during prolonged standing. freely flowing cutaneous blood.
Transport and analysis of specimen should be within ___ of collection.
Because electrodes are not stable over long periods of time, frequent calibration of ___, ___ and ___ is required:
Proper maintenance includes:
Good quality assurance includes: pH, PCO
2
, and PO
2
-meticulous care.
-adherence to the manufacturer's procedures.
-control of the equipment.
-proper collection and handling of specimens.
-the frequency with which maintenance should be maintenance = volume of analysis performed.
-proper maintenance of the instrument.
-use of control materials.
-verification of electrode linearity.
-checking of barometer accuracy.
-accurate measurement of temperature.
4
Chem PPT Flashcards, Unit 3
External quality assurance (proficiency testing) mandated by:
A hormone is:
CLIA'88
Hormones are produced at one site in the body and exert their action(s):
Paracine action is: a chemical substance produced in the body by an organ, cells of an organ, or scattered cells that has a specific regulatory effect on the activity of an organ or organs. at distant sites through what is called the endocrine system.
Autocrine action is: action of certain hormones that exert their effect locally on nearby cells. action of certain hormones that exert their effects on the cells of origin.
Polypeptide or Protein Adrenocorticotropic hormone (ACTH), insulin, parathyroid hormone (PTH) are examples of
___ or ___ hormones
This class of hormone is soluble in:
This class of hormone has a half-life of ___ to
___.
This class of hormone initiates response by:
Water/blood
≤10 to 30 minutes
Binding to cell membrane receptors and exciting the second messenger system.
Steroid Cortisol and estrogen are 2 examples of _____ hormones.
Steroid hormones are hydrophobic and water insoluble.
True
True Steroid hormones circulate in plasma, reversibly bound to transport proteins with only a small fraction free or unbound and available to exert physiological action.
What is the half-life of steroid hormones? 30-90 minutes
How do steroid hormones enter the cell?
What are 2 examples of amino acid-related
Passive diffusion
Thyroxine and catecholamines hormones?
Amino acid-related hormones are water soluble. True
True Amino acid-related hormones interact with membrane associated receptors and use a second messenger system.
Amino acid-related hormones circulate in plasma bound to ______ or _______
Proteins, free
Gonadal Estrogen and androgen are examples of ______ hormones.
What is the ability or tendency of an organism or cell to maintain internal equilibrium by adjusting its physiological processes?
In response to a glucose load, _____ is released from the ______.
Homeostasis
Insulin, pancreas
5
Chem PPT Flashcards, Unit 3
What is responsible for regulating the dispersal of glucose into cells for the metabolism necessary to produce energy?
What are the counter regulatory hormones that regulate glucose concentration?
What are some examples of incretins?
What is GLP-1?
Insulin
Glucagon, cortisol, epinephrine, growth hotmone and incretins
GLP-1 and GIP
Glucagon-like peptide 1
What is GIP?
What is CaSR?
The CaSR on the parathyroid gland recognizes the circulating level of ionized calcium and regulates the synthesis and secretion of ____.
What is PTH?
Gastic inhibitory pepide
Calcium-sensing receptor
PTH
Parathyroid hormone
PTH enhances renal tubular reabsorption of ? Calcium
PTH catalyzes the synthesis of renal hormone Calcitrol
_____ to increase intestinal absorption of calcium.
Aldosterone, renin, vasopressin The metabolism of water and electrolytes is regulated by _____, _______, and ______.
Where is aldosterone produced? Adrenal gland
Where is renin produced?
Where is vasopressin produced?
What is the posterior pituitary gland called?
Vasopressin is an _______ hormone.
Kidney
Posterior pituitary gland neurohypophysis
Antidiuretic
What is the role of hormone receptors? The unique or specific action of a hormone on its target tissue is a function of the interaction between the hormone and its receptor
What are the two types of hormone receptors? Cell surface receptors
Intercellular receptors
G-protein-coupled receptors (GPCR) What is a large superfamily of membrane receptors whose intracellular effects are mediated by G proteins?
What are a family of proteins involved in transmitting chemical signals outside the cell, and causing changes inside the cell?
What are characterized by a hormone binding domain, DNA-binding domain and an amino terminal variable domain?
Measurement of hormones
Guanine-nucleotide-binding proteins (G proteins)
Intracellular receptors
-Bioassay Techniques
-Receptor-Based Assay
-Immunoassay Techniques
-Instrumental Techniques
-Mass Spectrometry (coupled with gas and liquid chromatography)
-Matrix Assisted Laser
Desorption/Ionization (MALDI)
6
Chem PPT Flashcards, Unit 3
What is a monoamine, an organic compound that has a catechol(benzene with two hydroxyl side groups) and a side-chain amine?
Where is Catecholamines produced?
Catecholamine (CA)
Catecholamines are produced mainly by the chromaffin cells of the adrenal medulla and the postganglionic fibers of the sympathetic nervous system
Dopamine Which Catecholamines acts as a neurotransmitter in the central nervous system, is largely produced in neuronal cell bodies in two areas of the brainstem: the substantia nigra and the ventral tegmental area?
Where does Catecholamines derived from?
Included among catecholamines are:
Fight-or-flight response
Amino acid tyrosine epinephrine (adrenaline), norepinephrine
(noradrenaline) and dopamine ; all of which are produced from phenylalanine and tyrosine
Release of the hormones epinephrine and norepinephrine
Adrenal medulla Epinephrine (adrenaline) functions as a hormone released by the
What is a monoamine neurotransmitter, biochemically derived from tryptophan?
Where does Serotonin primarily found?
Serotonin or 5-hydroxytryptamine ( 5-HT
Gastrointestinal tract (GI tract), blood platelets, and the central nervous system
(CNS) of animals, including humans
Approximately 90% How many percent of the human body's total serotonin is located in the enterochromaffin cells in the GI tract, where it is used to regulate intestinal movements?
When platelets bind to a clot, they release?
Once released by the platelets, serotonin serves as a ______ and helps regulate homeostasis and blood clotting.
Serotonin also serves as a ______ for some type of cells, possibly giving it a roll in wound healing.
Serotonin
Vasoconstrictor
Growth factor
What is serotonin mainly metabolized into?
5-HIAA (hydroxyindoleacetic acid) is exerted by?
Phenylethylamines with hydroxyl groups on positions three and four of the benzene ring and on ethylamine sidechain on position one are called.
5-HIAA (hydroxyindoleacetic acid)
The kidneys
Catecholamines
Serotonin, norepinephrine,dopamine, and epinephrine are all types of?
Catecholamines
This catecholamine is acted upon by the pineal Serotonin
)
7
Chem PPT Flashcards, Unit 3 gland to produce melatonin.
Tyrosine is converted to 3,4dihydroxyphenylalanine (L-dopa) by the enzyme...
Conversion of L-dopa to ____is catalyzed by aromatic-L-amino acid decarboxylase
Dopamine formed is further converted to
_______by dopamine β-hydroxylase further conversion of norepinephrine to
_______is through the presence of phenylethanolamine and N-methyltransferase in the adrenal medullary chromaffin cells tyrosine hydroxylase dopamine norepinephrine epinephrine
_____is converted to 5-hydroxytryptophan by the enzyme _______.
Conversion of 5-hydroxytryptophan to ____is catalyzed by aromatic-L-amino acid decarboxylase
______is synthesize from ______in the pineal gland by the serotonin-N-acetyltransferase and by the hydroxyindole-O-methyltransferase
Monoamines include…
Tryptophan, tryptophan hydroxylase
Serotonin
Melatonin, serotonin
How are monoamines stored
Epinephrine, norepinephrine, serotonin, & dopamine
Monoamines are stored in secretory granules in equilibrium with the surrounding cytoplasm
How are monoamines released Monoamines are released from secretory vesicles into the extracellular space through the process of exocytosis an influx of calcium and acetylcholine The process of releasing monoamines is stimulated by?
In which nervous system does the presence of monoamine oxidase (MAO) lead to the conversion of norepinephrine to 3,4-dihydroxyphenylglycol (DHPG)? Parasympathetic or
Sympathetic
DHPG is then metabolized by cathechol-Omethyltransferase (COMT) in the extraneuronal tissues to
What is the primary end product of epinephrine and norepinephrine metabolism?
Where is vanillylmandelic acid produced?
Sympathetic nervous system.
3-methoxy-4-hydroxyphenylglycol (MHPG).
Vanillylmandelic acid (VMA)
What is the enzyme required for conversion of
MHPG to VMA
Liver
Alcohol dehydrogenase
What is the product of serotonin deamination? 5-hydroxyindoleacetic acid (5-HIAA)
What is the major urinary excretion product of 5-hydroxyindoleacetic acid (5-HIAA) serotonin metabolism?
Which system does norepinephrine regulate? The sympathetic nervous system and overall
8
Chem PPT Flashcards, Unit 3 state of attention and vigilance
Which processes are influenced by dopamine? 1) Reward seeking behavior
2) Initiation and maintenance of movement
3) Processing of sensory signals
4) Regulation of hormonal release.
Which processes are regulated by serotonin?
How does the sympathetic nervous system operate?
What does the sympathetic nervous system regulate?
1) Memory
2) learning
3) feeding behavior
4) sleep patterns
5) thermoregulation
6) pain modulation
7) cardiovascular function
8) Regulation of pituitary hormones.
It operates below the level of consciousness in controlling the physiological function of many organs and tissues of the body.
It regulates cardiovascular function in response to postural, exertional, thermal and mental stress
What happens when the sympathetic nervous system is activated?
Activation increases heart rate, constricts peripheral blood vessels, dilates skeletal arterioles, and elevates blood pressure.
Adrenal Medullary system Which system is characterized by the presence of numerous catecholamine storage granules?
These turn brown when exposed to what?
What does this color change indicate?
What are these cells/granules called?
Adrenal Medullary system secretes what?
What processes are stimulated by the release of epinephrine?
How does epinephrine raise glucose concentrations?
What other function does epinephrine affect?
1) Potassium bichromate solution
2) Ammoniacal silver nitrate
3) Osmium tetroxide
Oxidation and polymerization of epinephrine and norepinephrine.
Chromaffin cells/chromaffin granules
Epinephrine
1) Lipolysis
2) Ketogenesis
3) Thermogenesis
4) Glycolysis
Stimulates glycogenesis and gluconeogenesis
What is dopamine?
Pulmonary function causing the dilation of airways
A neurotransmitter produced in sympathetic nerves and the adrenal medulla
In the kidneys, what does dopamine regulate? Sodium excretion
Where else are dopamine metabolites produced? In the GI tract
What is the major urinary dopamine metabolite? Homovanillic acid (HVA)
What is the Enteric nervous system (ENS)? An independent and integrated system of neurons and supporting cells located in the
9
Chem PPT Flashcards, Unit 3
The ENS is composed of what two networks?
How is the ENS connected to the CNS? gastrointestinal tract, gallbladder and pancreas
1) Myenteric plexus
2) Submucous plexus.
It is connected by intrinsic sympathetic and parasympathetic motor neurons and by spinal
What are examples neuroendocrine tumors that produce catecholamine? and vagal sensory neurons.
1) Pheochromocytomas
2) Paragangliomas
3) Neuroblastomas
What are serotonin producing tumors? Carcinoid tumors
Pheochromocytomas occur within what gland? Adrenal gland
Adrenal gland Paragangliomas occur outside what gland that is commonly referred to as extra adrenal pheochomocytoma’s?
Hypertension, headaches, palpitations, diaphoresis, pallor, nausea, attacks of anxiety and generalized weakness are all symptoms of what referring to the adrenal gland?
Patients with higher risk for pheochromocytoma include those with a
_____ predisposition to the tumor and finding of an ______ ______ during a routine abdominal imagine procedure.
In terms of pheochromocytoma and paraganglioma, this type of diagnosis are based on evidence of excess production of catecholamines by measurements id metanephrines in urine or plasma.
Pheochromocytoma and Paragangliomas hereditary adrenal mass
Biochemical Diagnosis
15%
35%
Nueroblastoma
Presence of most pheochromocytomas are __% benign of adrenal tumors and ___% of extraadrenal tumors are malignant.
This is a neoplasm that id derived from primordial neural crest cell of the sympathetic nervous system.
Neuroblastoma causes sporadic ______ cancer, common malignancies in the first year of life
Mutations of neuroblastomas activate in the
______ kinase domaine of the anapestic lymphoma kinase oncogene account for most cases of heredity
Neuroblastomas have Variable biological behavior but most are ____?
____ and ____ are most widely used for diagnosis of neuroblastoma.
Diagnosis of neuroblastoma is diagnosed pediatric tyrosine aggressive
HVA and VMA catecholamine
10
Chem PPT Flashcards, Unit 3 mainly on measurements of
_______ metabolites.
Gastroenteropancreatic neuroendocrine and carcinoid tumors are tumors from the
____________ cells.
Gastroenteropancreatic neuroendocrine and carcinoid tumors are most common in the _____ or lungs, _____ or jejunum, _____ and rectum.
Gastroenteropancreatic neuroendocrine and carcinoid tumors usually appear in ____
(pediatrics, older adults)
These tumors are characterized by large quantities of serotonin.
In carcinoid tumors, ______ is converted to serotonin and is stored in circulatory granules and in platelets.
What type of test result elevations are seen in wide ranges of serotonin rich foods or medication?
What type of false result can be caused by alcohol and other drugs?
Neuroendocrine tumors derive from enterochromaffin cells of the respiratory tracts and _______. Bowel obstruction and abdominal pain are presented. enterochromaffin bronchus ileum rectum older adults
Carcinoid tumors
5-HTP
False-positive
False-positive gastrointestinal
Biochemical diagnosis of carcinoids depends mainly on measurements serotonin, serotonin metabolites (____), and the serotonin precursor
(____) in urine, plasma, whole blood and platelets.
What type of false result are a common problem resulting from dietary influences.
For catecholamines and their metabolites, serotonin and its metabolites and urinary or plasma metanephrines are determined by laboratory what type of methods?
What kind of anticoagulant should be used for whole blood measurement of serotonin?
Aliquots of blood is removed to count what?
(5-HIAA)
(5-HTP)
False-positive
Analytical methods
How are blood serotonin samples stored?
Platelet rich plasma is prepared from whole blood using what speed on the centrifuge?
Why should platelet rich plasma be prepared within 1 hour after collection and be placed on ice?
EDTA, gently mixed and placed on ice and transferred to a storage tube
Platelets
Frozen at -20C within 2 hours after collection
Low-speed
To prevent lowering of serotonin concentration
Plasma and platelets should be stored at what -20C
11
Chem PPT Flashcards, Unit 3 temperated?
Plasma and platelets are analyzed within how many weeks after collection?
How are 24-hour urine samples for serotonin and 5-HIAA collected?
1-2 weeks
In 2 L brown polypropylene bottles each containing 250 mg of sodium metabisulfite and EDTA as preservatives
What pH lever are urine samples acidified to? pH 4
Acetic acid What is used to acidify urine samples before freezing?
True or False?
Urine samples don’t have to be refrigerated during collection.
False. Urine specimens should be refrigerated during collection.
How do drugs affect monoamine systems?
Give an example of a drug from the above question.
Drugs that affect monoamine systems are the major reason of false-positive results for the measurements of what?
What kind of dietary food sources should you avoid 3-4 days before and during urine collection?
By Inhibiting monoamine reuptake
Tricyclic anti-depressants
Norepinephrine and normetanephrine
Dietary sources of 5-hydroxyindole such as walnuts, avocado, bananas, eggplants, pineapples, plums, and tomatoes.
True True or False?
Metanephrines and methoxytyramine are present in plasma and urine
How is urinary and plasma fractionated metanephrines measured?
What kind of preparation step is taken for measurement of urinary and plasma fractionated metanephrines?
By LC-MS/MS
An ion exchange chromatography
What detection methods are used for plasma catecholamines?
What is LC-EC?
LC-EC
What does VMA stand for?
What does HVA stand for?
What is the major end product of epinephrine and norepinephrine metabolism?
HVA is the major end product of the metabolism of what?
Urinary VMA and HVA are used for the diagnosis of what?
What methods are used to detect VMA and
HVA?
Liquid chromatography with electrochemical detection
Vanillylmandelic Acid
Homovanillic Acid
VMA
Dopamine
Neuroblastoma
Gas or liquid chromatography and LC-
MS/MS
What does 5-HIAA stand for? 5-hydroxyindoleacetic acid
Serotonin and 5-HIAA are measured in what? Whole blood, platelet rich plasma, or patelet pellets
12
Chem PPT Flashcards, Unit 3
What can serotonin and 5-HIAA identify?
What is used to measure serotonin and 5-
HIAA?
Fill in the blank:
Water soluble vitamins are retained (less/more) and excreted (less/more) in the urine
Water soluble vitamins can function as what?
Give 2 examples
True or False?
Fat soluble vitamins are soluble in organic solvents
Tumors deficient in aromatic amino acid decarboxylase
Liquid chromatography with fluorometric or electrochemical detection, HPLC
Less; more
Coenzymes; B-complex group vitamins and
Vitamin C
True
Fat soluble vitamins are absorbed, transported, and stored for (shorter/longer) periods of time
Give 4 examples of fat soluble vitamins
What are sources of vitamin A?
What are sources of beta-carotene?
Retinol is principally stored as what?
Where is retinol obtained?
Provitamin A carotenoids are obtained from what?
What is a major function of vitamin A?
What are other functions? include its role in reproduction, growth, embryonic development and immune function.
What else does it provides protection against? cancer by blocking tumor promotion,
Deficiency of Vitamin A causes? inhibiting proliferation, inducing apoptosis and inducing differentiation
Night blindness (nyctalopia), Xerophthalmia,
Keratomalacia, Dryness /roughness of the skin, papular eruptions and follicular hyperkeratosis
What is the laboratory assessment?
What is the chemical assessment?
What are sources of vitamin D?
When does the body make vitamin D?
What is vitamin D’s main circulating form?
What is its biologically more active form?
What is vitamin D3?
Longer
Vitamins A, D, E, K
Eggs, meat, and dairy
Green leafy vegetables, and vibrant colored fruits and vegetables. of retinyl esters (palmitate) and includes dietary carotenoids such as α-carotene, βcarotene, and β-cryptoxanthin from liver, other organ meats, fish oils, full cream milk, butter and fortified margarines. yellow or orange pigment fruits and green leafy vegetables
Good vision
Measurement of RBP (retinol binding protein) and transthyretin (thyroxine-binding prealbumin) by nephelometry
Carr-Price and Neeld-Pearson methods
Cheese, margarine, butter, fortified milk, healthy cereal, fatty fish
When exposed to sunlight
25 hydroxyvitamin D [25(OH)D],
1, 25 dihydroxyvitamin D [1,25 (OH)2D], the parent compound of the naturally
13
Chem PPT Flashcards, Unit 3
What is vitamin D2?
What are ways Vitamin D may be acquired?
Vitamin D2 and vitamin D3 are metabolized to what?
Metabolites are further metabolized by?
How does1,25(OH)2D act on intestine ?
How does 1,25(OH)2D act on bones? occurring family and is produced in the skin from 7 dehydrocholesterol on exposure to the
UV B portion of sunlight the parent compound of the other vitamin D family, manufactured by irradiation of ergosterol produced by yeast by exposure of skin to sunlight or ingestion of foods containing vitamin D, primarily fish liver oils, fatty fish, egg yolks and liver
25(OH)D2 and 25(OH)D3, respectively, in the liver by vitamin D-25-hydroxylase. in the kidneys and also in the placenta of pregnant women by 25(OH)-D-1αhydroxylase.
? is 1,25 (OH)2D What is the biologically most active form of vitamin D
What is the main circulating form of vitamin D? 25(OH)D.
How vitamin D plays in control of calcium Hypercalcemia reduces 25(OH)-D-1αlevels? hydroxylase activity and production of
1,25(OH)D.
Hypocalcemia increases the synthesis of 1,25
(OH)2D by increasing 25(OH)-D-1αhydroxylase activity
How vitamin D plays in control of phosphate? Hyperphosphatemia reduces 25(OH)-D-1αhydroxylase activity and production of
1,25(OH)D.
Hypophosphatemia increases the synthesis of
1,25 (OH)2D by increasing 25(OH)-D-1αhydroxylase activity
intestine, bone, kidney, and parathyroid. Where are calcium and phosphate concentrations in serum that are maintained by the actions of 1,25(OH)2D?
How does 1,25 (OH)2D act? 1,25 (OH)2D reduces 25(OH)-D-1αhydroxylase activity and production of
1,25(OH)D.
It also induces 25(OH)D-24-hydroxylase, an enzyme producing 24,25-dihydroxyvitamin D
(24,25 [OH]2D), which is the most prevalent dihydroxylated vitamin D form in serum. The activity of this enzyme may reduce the formation of biologically active 1,25(OH)2D.
1,25(OH)2D stimulates calcium absorption
1,25(OH)2D increases bone resorption and increases the circulating concentration of bone
14
Chem PPT Flashcards, Unit 3
How does 1,25(OH)2D act on kidney?
How does 1,25(OH)2D act on parathyroid?
What does measurement of 25(OH)D use?
What does measurement of 1,25(OH)2D use?
What are measurements of vitamin D metabolites? alkaline phosphatase (BALP), and the noncollagenous bone protein osteocalcin (OC)
1,25(OH)2D inhibits its own synthesis and stimulates its metabolism
1,25(OH)2D acts directly to inhibit the synthesis and secretion of PTH
Useful in evaluating hypocalcemia, vitamin D status, and bone disease
Useful in detecting in adequate or excessive hormone production in the evaluation of hypercalcemia, hypercalciuria, hypocalcemia and bone and mineral disorders
1. Competitive Protein Binding Assay
(CPBA)
2. Immunoassay
3. UV absorbance after separation by High
Performance Liquid Chromatography (HPLC)
4. Liquid Chromatography-Tandem Mass
Spectrometry (LC-MS/MS)
What is the nutrition term for the group of vitamin E?
Where isVitamin E absorbed in the human body?
How vitamin E is secreted?
These are tocopherols and tocotriennols.
How are tocopherols and tocotriennols found? principal sources of dietary vitamin E are oils and fats, particularly with germ oil and
What is the major form of vitamin D in many plant seeds? sunflower oil, grains and nuts
γ-tocopherol
Vitamin E is absorbed from the small intestines in the presence of bile
How isVitamin E excreted?
It is secreted in chylomicron particles which are then transported to the peripheral tissue, mainly adipose tissue, with the aid of lipoprotein lipase (LPL)
The liver takes up the chylomicrons where the
α-tocopherol is incorporated into VLDL
Vitamin E is excreted via the bile and in the urine as tocopheronic acid and its βglucuronide conjugate
What is Vitamin E necessary for? Vitamin E is necessary for neurological and reproductive functions, protection of red cells from hemolysis, prevention of retinopathy in premature infants and inhibition of freeradical chain reactions of lipid peroxidation.
What is Vitamin E? Vitamin E is an antioxidant that acts as a scavenger for molecular oxygen and free radicals and has a role in cellular respiration
What are the risks of deficiency of vitamin E? Deficiency of vitamin E are generally
15
Chem PPT Flashcards, Unit 3
Excess of vitamin E? observed in premature and low birth weight infants
It is primarily due to dietary supplementation and may cause deficiency of fat soluble vitamins D and K by competitive absorption.
High performance liquid chromatography
(HPLC)
What is the method of choice to quantify tocopherols in serum?
What is Phylloquinones (Vitamin K1 type)?
Synthesize in plants
What is Menaquinones (Vitamin K2 type) Bacterial origin
How does vitamin K get destroyed?
It Got destroyed by alkaline solution and reducing agents and are sensitive to ultraviolet light
What are the Dietary sources of phylloquinones?
What are the Dietary sources of menaquinones? Dietary sources of menaquinones our cheese, eggs and milk products
Where is vitamin K absorbed from?
Dietary sources of phylloquinones are green vegetables, margarines and plant oils.
Vitamin K is absorbed from the small intestines in the presence of bile, bound to chylomicrons.
Where do the traces of urinary metabolites of vitamins of K-1 and K2 appear in?
What does vitamin K promote? What is vitamin
K required for?
Only traces of urinary metabolites of vitamins
K-1 and K2 appear in urine
Vitamin K promotes clotting of the blood and is required for the conversion of several clotting factors and prothrombin.
What is the risk factor of vitamin K?
What are the labrotory assessments for vitamin
K?
What do the Dietary sources of vitamin B1 include?
Risk of vitamin K deficiency is increased in fat malabsorption states such as bile duct obstruction, cystic fibrosis, chronic pancreatitis and liver disease. Risk is also increased by the use of drugs that interfere with vitamin K metabolism such as coumarin anticoagulants (warfarin) and some antibiotics
(cephalosporin).
Laboratory Assessment includes:
Prothrombin time (PT) determination direct measurement of plasma phylloquinone by High Performance Liquid Chromatography
(HPLC)
Dietary sources include unrefined cereal grains, breakfast cereals and enriched flour.
Where does absorption of Vitamin B1 Occur? Absorption occurs primarily in the proximal small intestines.
Where is vitamin B1 Stored? About half of the body stores are found in skeletal muscles, with much of the remainder in the heart, liver, kidneys and nervous tissue including the brain which contains most of the triphosphate.
16
Chem PPT Flashcards, Unit 3
What is the function of Thiamine in Vitamin
B1?
What are the two general reactions of Thiamine in Vitamin B1?
What is thiamine necessary for?
What does the deficiency results to? What are the symptoms?
What is the deficiency in thiamine due to?
What is the labrotory assessment for vitamin
B1?
What is an essential component of Riboflavin?
(Vitamin B2)
What do the dietary sources include in Vitamin
B2
What is Vitamin B2 absorbed in?
Thiamine functions to form the coenzyme thiamine pyrophosphate (TPP ), which is required for the essential decarboxylation reactions catalyzed by the pyruvate and 2oxoglutarate complexes.
The two general reactions are:
1. Oxidative decarboxylation of 2-oxo acids catalyzed by dehydrogenase complexes
2. Formation of 2-ketols (ketoses) as catalyzed by transketolase
Thiamine is necessary for the metabolism of carbohydrates, fats and alcohol.
Deficiency results to beriberi, involving the nervous and cardiovascular systems.
Symptoms include mental confusion, anorexia, muscular weakness, ataxia, peripheral paralysis, opthalmoplegia, edema
(wet beriberi), muscle wasting (dry beriberi), tachycardia and an enlarged heart.
Deficiency of thiamine is due to:
1. Inadequate intake caused by diets largely dependent on milled, non-enriched grains
2. Ingestion of raw fish containing microbial thiaminases
3. Chronic alcoholism
4. Those receiving total parenteral nutrition
(TPN) without adequate thiamine supplementation
5. Elderly patients taking diuretics
6. Patients undergoing long-term renal dialysis
Laboratory Assessment:
Measurement of transketolase
•
Brin procedure
Direct measurement of circulating thiamine in plasma, erythrocytes or whole blood using
High Performance Liquid Chromatography
(HPLC)
Is an essential component of flavin adenine dinucleotide (FAD) and flavin mononucleotide (FMN), coenzymes that are involved in many redox reactions.
Dietary sources include liver, kidney, heart and milk.
It is primarily absorbed in the proximal small intestines in the presence of bile, tightly
17
Chem PPT Flashcards, Unit 3
Does vitamin B2 trace in urine?
What are signs of riboflavin deficiency?
What are the 4 laboratory assessment for riboflavin?
What are the 3 natural forms of vitamin B6?
What are the dietary sources of Vitamin B6?
What is the major coenzyme of vitamin B6?
What is the main catabolite excreted in urine from vitamin B6?
What are the steps for laboratory assessment for vitamin B6? bound to immunoglobulins.
Because little storage of riboflavin occurs, urinary excretion reflects dietary intake.
Deficiency of riboflavin is characterized by sore throat, hyperemia, edema of the pharyngeal and oral mucous membranes, cheilosis, angular stomatitis, glossitis
(magenta tongue), seborrheic dermatitis and normochromic, normocytic anemia.
Laboratory Assessment:
1. Determination of urine riboflavin excretion
2. A functional assay using the activation coefficient of stimulation of the enzyme glutathione reductase by FAD
3. Direct measurement of riboflavin or its metabolites in plasma or erythrocytes
4. HPLC combined with fluorometric detection is the method of choice
Three natural forms of vitamin B6:
pyridoxine [pyridoxol] (PM)
pyridoxamine (PM)
pyridoxal (PL)
All three are converted to pyridoxal phosphate, which is required for synthesis, catabolism and inter-conversion of amino acids
Dietary sources include meat, poultry and fish, used certain seeds, bran, bananas and fortified ready-to-eat cereals.
The major coenzyme (PLP pyridoxal-5’phosphate) used by the PLP dependent enzymes that are involved in amino acid metabolism.
The main catabolite excreted in urine is 4pyridoxic acid (4-PA)
Laboratory Assessments :
1.Measurement of PLP (pyridoxal-5’-phosphate) in plasma or red cells
2. Measurement of its metabolite, 4-pyridoxic acid
(4-PA) in urine or plasma
3. Measurement of the activity and activation coefficient of red cell aminotransferases (aspartate and alanine)
4. Tryptophan load metabolite excretion tests
5. High Performance Liquid Chromatography
(HPLC) with fluorescence detection
18
Chem PPT Flashcards, Unit 3
What are some characteristics of vitamin B12? A water-soluble hematopoetic vitamin that is required for the maturation of erythrocytes.
Cyanocobalamin is a stable compound that forms dark red, needlelike crystals.
It is the reference compound used to calibrate serum cobalamin methods
The predominant physiological form in serum is methylcobalamin , whereas that in cytosols is adenosylcobalamin.
Cyanocobalamin is gradually destroyed on exposure to light
What are the dietary sources of Vitamin B12? Dietary sources are meat and meat products, dairy products, fish and shellfish and fortified ready-to-eat cereals.
Where is vitamin B12 secreted?
Where will excess vitamin B12 be excreted?
Where is the greatest loss of vitamin B12 occurring?
What is vitamin B12 deficiency associated with?
It is continually secreted in the bile, but most is reabsorb and available for metabolic functions.
If circulating vitamin B12 concentrations exceed the binding capacity of the blood, the excess will be excreted in the urine.
In most circumstances, the greatest looses of vitamin B12 occur through the feces.
Deficiency of vitamin B12 is associated with megaloblastic anemia and neuropath y.
The most common cause of vitamin B12 deficiency is pernicious anemia .
What are the hematological effects of vitamin
B12 deficiency?
What is the laboratory assessment of vitamin
B12 ?
The hematological effects of vitamin B12 deficiency are indistinguishable from those of folate deficiency. The classic morphological changes in the blood are hypersegmentation of neutrophils, macrocytosis, anemia, leukopenia and thrombocytopenia with megaloblastic changes in bone morrow accompanying peripheral blood changes.
Laboratory Assessment:
Indirect tests include:
1. Assays for urinary and serum concentrations of methylmalonic acid
2. Assays for plasma homocysteine
3. The deoxyuridine suppression test
4. Vitamin B12 absorption tests
Direct tests include:
1. Microbiological Competitive Protein
Binding (CPB)
2. Immunoassay
19
Chem PPT Flashcards, Unit 3
What are the characteristics of Vitamin C?
What are Laboratory assessment for VITAMIN
C- ASCORBIC ACID?
Serves as a reducing agent in several important hydroxylation reactions in the body
One of the most effective watersoluble antioxidants in biological fluids
Dietary sources include citrus fruits, berries, melons, tomatoes, green peppers, broccoli,
Brussels sprouts and leafy green vegetables.
The gastrointestinal absorption is regulated by a combination of sodium dependent active transport at low concentrations, and simple diffusion at high concentrations.
1. Direct measurement of plasma, urine, or tissue concentrations of ascorbic acid or total vitamin C.
2. Measurement using ascorbate oxidase enzyme
3. High Performance Liquid Chromatography
(HPLC) methods
What are good sources of biotin?
What characteristic Biotin has?
Liver, kidney, pancreas, eggs, yeast and milk.
Biotin in the diet is largely protein bound and digested by gastrointestinal enzymes.
To serve as a cofactor for carboxylation What is the principal biochemical function of
Biotin or Vitamin H?
In what disorders Biotin deficiency may be seen? reactions.
TPN (total parenteral nutrition) without biotin supplementation and in patients with a genetic deficiency of biotinidase.
Lactobacillus plantarum What test organism is used in microbiological assay for Vitamin H?
What is considered to be a better indicator of biotin status?
Urinary excretion of biotin and
3-hydroxyisovaleric acid
What is the function of Folate and folic acid? functions as coenzymes in the processing of one carbon units
Folate and Folic acid are derived from ……….. and the principal form is ……………... pteroic acid, 5-methyltetrahydrofolate
What are food source for Folic acid? Liver, spinach, and other dark green leafy vegetables, legumes such as kidney and Lima beans and orange juice.
What causes deficiency of folate? Absence of intestinal microorganisms, poor intestinal absorption, insufficient dietary intake (chronic alcoholism), and excessive demands is in pregnancy, liver disease and malignancies, administration of anti-folate
20
Chem PPT Flashcards, Unit 3
What is the major clinical manifestation of folate deficiency? drugs and anticonvulsant therapy leading to increase folate requirements.
Megaloblastic anemia (characterized by large, abnormally nucleated erythrocytes in the bone morrow)
What are laboratory assessment for folic acid? Measurement of folate concentration using C room erythrocyte or whole blood the specimen
CPB (Competitive Protein Binding) assays
What the term Niacin refer to? 1. Nicotinic acid (pyridine-3-carboxylic acid)
2. it's amide niacinamide (nicotinamide)
3. Derivatives that show the same biological activity as nicotinamide
What are sources of Niacin?
What is characterization of pellagra?
Yeast, lean meats, liver, poultry, milk, canned salmon and several leafy green vegetables, corn and wheat.
Nicotinamide What is the main circulating form of Niacin in the plasma after absorption or release from hydrolyzed liver NAD?
In what form Excess niacin is excreted in liver? N-methylnicotinamide (NMN)
Name the Vitamin that is essential for the Niacin coenzymes NAD and NADP?
What is the function of Nicotinic acid, when used as a pharmaceutical agent?
It has important anti-atherogenic properties. It effectively lowers triglycerides, raises HDL cholesterol, and shifts LDL particles to a less atherogenic phenotype.
What disease is result of Niacin deficiency? Pellagra is the classic deficiency disease associated with niacin and tryptophan
Chronic wasting disease presentation associated with dermatitis, dementia and diarrhea.
What are laboratory assessments for NIACIN and NIACINAMIDE?
What Vitamin is a component of Coenzyme A? Pantothenic acid
What is the most common commercial synthetic Calcium salt form of pantothenic acid?
What are the source of Pantothenic acid? It is widely distributed in foods, mostly within
Co-A containing compounds like animal sources, legumes, whole-grain cereals, egg yolk, kidney, liver and yeast.
What is the function of Pantothenic acid?
What are Pantothenic Acids two major metabolic roles?
Urinary measurement of N'methylnicotinamide and N'-methyl-2pyridone-5-carboxamide using HPLC.
It is required for the metabolism of fat, protein, and carbohydrate via the citric acid cycle.
It is part of coenzyme A and is a prosthetic group of the acyl-carrier protein, ACP.
21
Chem PPT Flashcards, Unit 3
What methods are used in determining the whole blood or urine concentrations of
Pantothenic Acid?
What are trace elements?
What are referred to as trace and ultratrace elements?
What type of specimens are tested for trace elemeants?
What types of contamination should be avoided?
Is it proper procedure to remove white cells and platelets from blood before testing for trace elements?
Which variables may affect trace element determination?
Is knowledge of any acute phase reactions required before testing for trace elements?
Is there a possibility of contamination wich containers made of rubber, cork, or colored plastics?
What type of tube should be used when testing trace elements in blood plasma?
What type of tube should be used when testing trace elements in blood serum?
What type of tube should be used when testing ultratrace metals (Mn,Cr)?
How are containers cleaned?
What type of tube should be used when testing trace elements in urine?
By microbiological assay, radio immunoassay, gas chromatography, gas chromatography mass spectrometry and a stable isotope dilution assay.
Inorganic molecules found in human and animal tissues in milligrams per kilogram amounts or less.
Those present in body fluids (µg/dL) and in tissues (mg/kg) are referred to as trace elements, and those of found at ng/dLor µg/kg as “ultratrace elements.”
Specimens commonly submitted include whole blood, plasma, serum, or anybody fluid or tissue.
Contamination from environmental pollution, cosmetics, shampoos must always be avoided.
No, separation of white cells and platelets in whole blood before trace element analysis is subject to serious problems of contamination.
Variables that can affect trace element determination include age, sex, ethnic origin, time of sampling in relation to food intake, time of day, history of medication and tobacco usage.
Yes, knowledge of the extent of any acute phase reaction is required.
Yes, avoid contamination with sample containers made up of rubber, cork and colored plastics.
For blood plasma, plastic tubes with lithium heparin as an anticoagulant are suitable for most analyses.
For blood serum, plain glass containers have been used.
For the ultratrace metals (Mn, Cr), special arrangements have to be made to collect blood via plastic cannulae or siliconized steel needles, and then the sample is placed into acid washed containers.
It is a good practice to run dilute acid blanks through all containers and collection systems to ensure that all batches remain as free from contamination as possible.
For 24 urine collection, polyethylene bottles with glacial acetic acid should be used as a
22
Chem PPT Flashcards, Unit 3
What methodologies are used to test for trace elements?
What are speciation methods? preservative.
1. Spectrophotometry
2. Atomic Absorption Spectrophotometry
(AAS)
3. Inductively Coupled Plasma-Optical
Emission Spectrometry (ICP-OES)
4. Inductively Coupled Plasma Mass
Spectrometry (ICP-MS)
5. Accelerator Mass Spectrometry (AMS)
6. X-ray based techniques
Involve techniques to separate the chemical complexes of individual elements present in any particular medium. They are regarded as crucial for an understanding of the absorption, utilization, function of elements and problems of excess and potential toxicity.
Chromium What occurs naturally in various crystal materials?
How is Chromium used of and disposed?
What are some good sources of chromium?
What does Chromium do after ingestion?
It is a transitional element with many industrial uses and is discharged into the environment as industrial waste.
Good sources of chromium include processed meats, whole-grain products, green beans, broccoli and some spices
After absorption, chromium binds to plasma transferrin with an affinity similar to that of iron.
What physiological effect does Chromium have?
It functions to enhance the response of insulin receptors and potentiates kinase activity to normalize glucose and insulin levels.
What does a deficiency in Chromium lead to? Poor chromium nutritional status plays a role in impaired glucose tolerance, diabetes and
Does Hexavalent Chromium have any toxic effects? cardiovascular disease.
Hexavalent chromium is a recognized carcinogen, and industrial exposure to fumes and dusts containing this metal is associated with increased incidence of lung cancer, dermatitis and skin ulcers.
Blood plasma or urine should be used. What type of specimen should be used when testing directly for chromium?
What vitamin is cobalt an essential integral part of?
What can hip prostheses and increased exposure to cobalt lead to?
What are some dietary sources of copper?
Vitamin B12
High mean urinary cobalt concentrations
Organ meats, shellfish, nuts, whole grain cereals, and cocoa containing products
23
Chem PPT Flashcards, Unit 3
How is absorbed copper transported to the liver?
Bound to albumin in portal blood
What happens to absorbed copper in the liver? It is incorporated by hepatocytes into cuproenzymes and then exported in peripheral blood mainly as ceruloplasmin to tissue and organs.
What is the function of copper? It functions for energy production, connective tissue formation, iron metabolism, norepinephrine and serotonin metabolism, synthesis of melanin, antioxidant functions,
What does DMO stand for? and regulation of gene expression and intercellular copper handling.
Dopamine mono-oxygenase
What does DMO require as a cofactor for the conversion of dopamine to norepinephrine?
What is tyrosinase?
Copper
What diseases is copper deficiency associated with in infants?
A copper containing enzyme that is present in melanocytes and catalyzes the synthesis of melanin.
Menkes disease, Wilson disease, malabsorption syndromes, cardiovascular disease, anemia, and neuropathy
How is laboratory assessment of copper toxicity done?
What element is the most widely used of the pharmacologically beneficial trace elements?
Where are fluoride ions absorbed?
How is excess fluoride excreted?
How does toxicity occur in children?
By determination of plasma copper and ceruloplasmin levels
Fluoride
The stomach and the small intestines
In the urine
By the mottling of enamel in the erupting teeth of children, possibly caused by ingestion of fluoride containing toothpaste.
What sort of exposure to fluoride has resulted in sever bone abnormalities in adults?
How is fluoride level determined in drinking water and urine?
What is manganese bound to when present in biological systems?
What functions does manganese serve in the body?
What are some dietary sources of copper?
Occupational exposure to inhaled fluoride dust among cryolite workers during aluminum refining
Direct determination using fluoride specific electrode is performed.
Protein in the 2+ or 3+ valence state
Formation of connective and bony tissue, growth and reproductive functions, and carbohydrate and lipid metabolism. include whole-grain foods, nuts, leafy vegetables, soy products and tea
Non-specific What sort of enzyme activator does manganese act as?
What can deficiency in manganese result in? Impaired growth and reproductive function, skeletal abnormalities, impaired glucose
24
Chem PPT Flashcards, Unit 3 tolerance and impaired cholesterol synthesis.
How is manganese assessed in the laboratory? By measurement of nonhemolyzed whole blood manganese using plastic cannulae for phlebotomy.
What do molybdenum enzymes facilitate? Important carbon, nitrogen, and sulfur cycles.
What are some dietary sources of molybdenum? Peas, lentils and beans, grains and nuts.
Its incorporation into metalloenzymes What is the essential need for molybdenum based on?
How is molybdenum level assessed in the laboratory?
By measuring urate or sulfite in the urine as a means of confirming molybdenum cofactor disorders or possible molybdenum deficiency.
What element is a constituent of the enzyme glutathione peroxidase?
What is glutathione peroxidase believed to be closely associated with?
What do the most important biologically active compounds contain?
What is selenocysteine
How is selenium measured? glutathione peroxidase
Vitamin E and its function
Selenocysteine
Amino acid in which selenium is substituted for sulfur in cysteine.
What are some dietary sources of selenium?
What is seleniums major route of excretion?
Wheat and other cereal products
Urinary output
What does urinary output of selenium reflect? Recent dietary intake of selenium
What are some selenium dependent diseases associated with selenium deficiency?
Keshan disease, and Kashin-Beck disease, and also associated with thyroid function, immune function, reproductive disorders, mood disorders, inflammatory conditions, cancer chemoprevention and viral virulence.
Carbon furnace atomic absorption spectroscopy (CFAAS) is widely used to measure plasma and/or serum selenium. selenoprotein P What is the major selenium containing plasma protein?
How is selenoprotein P determined?
How can long-term dietary selenium intake be measured?
Next to iron, what is the most abundant trace element in the body?
What is it usually bound to in zinc rich foods like red meat and fish?
What is impaired in wound healing for people with zinc deficiency?
What is the function of zinc in sperm?
By immunological methods
Hair and nail selenium analysis
Zinc
Proteins
Wound Healing
Which factors help absorption of zinc in breast milk?
What are the clinical effects of ingestion of a zinc contaminated diet?
Maintain vitality and sperm motility
Picolinate and citrate
Abdominal pain, diarrhea, nausea, vomiting.
25
Chem PPT Flashcards, Unit 3
Why are plasma zinc determination preferred over zinc serum samples?
What are good sources of boron?
How is boron absorbed and secreted?
Because of possible zinc contamination from erythrocytes platelets and leukocytes during clotting and centrifugation.
Fruits, leafy vegetables, nuts and legumes.
It is sufficiently absorbed as boric acid and is efficiently excreted in the urine.
What is inductively coupled plasma- atomic emission spectrophotometry (ICP-AES) and an
ICP time-of-flight mass spectrometer (TOF-
MS) developed for?
Investigation of boron neutron capture in cancer therapy.
What is silicon primarily used for in the body? Structural Identity.
How is the laboratory assessment for silicon done?
By determination of healthy fasting plasma concentration of silicon.
How is vanadium found in neutral solutions? Metavanadate (V3)
How is vanadium helpful in treating diabetes? By reducing the requirement for insulin by activating the cellular response without the presence of insulin.
GF-AAS or ICP-AES How is plasma and urine concentrations are usually measured?
What is hemoglobin and what is it responsible for?
Hemoglobin is the oxygen-carrying pigment of the erythrocytes. It is for transportation of
O
2
from lungs to the body tissues, as well as
CO
2
from peripheral tissues to the lungs.
Where is hemoglobin formed?
What composes hemoglobin?
Hemoglobin is formed by the developing erythrocyte in the bone marrow. composed of two types of globins organized into four subunits
The heme chelates with the globin portion. How does the heme bind with the globin portion of hemoglobin?
How is hemoglobin classified? Hemoglobins are classified into different types, depending on the combination of the two sets of globin units
α-globins and β-globins
What are the most common types of globin units in adult humans?
What is a prosthetic group? A prosthetic group is any tightly-bound non-protein entity,that is essential for the structural and functional integrity of the protein.
What is the prosthetic group of hemoglobin? The heme group.
What composes the heme group?
What is the function of the function of the iron ion in hemoglobin?
Porphyrin ring, which is formed by the combination of four heterocyclic rings called pyrroles with an Fe
2+ ion in the center of the ring bound to the nitrogen of the pyrroles.
It is this central iron which provides the reversible binding to oxygen and carbon dioxide molecules
26
What is Hemoglobin?
What is oxyhemoglobin or carbaminohemoglobin?
What is deoxyhemoglobin?
What is oxyhemoglobin?
What is carboxyhemoglobin?
What is methemoglobin?
What is the adduct Hbs?
Where do you see carbamylated Hb?
How is glycated, glycosylated Hb if form?
What is fetal Hb (HbF)
Chem PPT Flashcards, Unit 3
What is the cause of methemoglobinemia?
What is Sulfhemoglobin?
•
Is the oxygen-carrying pigment of the erythrocytes that is formed by the developing erythrocyte in the bone marrow.
•
It is mainly responsible for the transportation of oxygen from lungs to the body tissues, as well as carbon dioxide from peripheral tissues to the lungs.
Each hemoglobin molecule is composed of two types of globins organized into four subunits
The heme is bound to an oxygen molecule or carbon dioxide molecule.
When the heme groups of hemoglobin molecule are not bound by any molecule.
Is the bright red color of blood?
When hemoglobin binds with carbon monoxide, compromising the oxygen-carrying ability of Hb.
Is the formed as a result of a change in oxidation state of the iron atom in heme from the normal ferrous state (2+) to ferric (3+) state, resulting in decreased oxygen-carrying ability.
Is caused by the presence of nitrate in well water.
Is commonly resulting from exposure to certain drugs, is formed when one or more oxygen atoms in the porphyrin rings of heme is replace by sulfur. Removal of the source of the chemical leads to restoration of normal
Hb.
Are formed by the attachment of molecule to the globin chins most commonly at the Nterminal amino acid, but may also occur anywhere along the globin chain.
In patients with end-stage renal disease, is formed by the attachment of urea.
Is form by the attachment of glucose to the Nterminus of the Beta globin chain, It ( HbA
1c
) is clinically important in diagnosis and monitoring of glycemic control in patients with diabetes mellitus
Is the main oxygen transport protein in the human fetus during the last seven months of
27
Chem PPT Flashcards, Unit 3
What is the Function of HbF? development in the uterus and persists in the newborn until roughly 6 months old.
Fetal hemoglobin differs most from adult hemoglobin in that it is able to bind oxygen with greater affinity than the adult form, giving the developing fetus better access to oxygen from the mother's bloodstream.
1.
Thalassemia’s and Hemoglobinopathies
What are the disease or disorders that related to
Hemoglobin?
What is Thalassemia’s?
What is hemoglobinopathies?
What are the etiology of Thalassemias?
What are the etiology of Thalassemias?
Are identified according to the globin chains in which there is a production deficiency:
α-thalassemia arise from defective α-globin chain production
β-thalassemia arise from defective β-globin chain production
δβ-thalassemia arise from deficiencies in production of both δ- and β-globin chains.
Are also classified by the extent of reduction in globin chain production and resultant anemia:
Single gene deletion = Silent α-thalassemia ( αα/α-)
Two gene deletion = α-thalassemia trait or αthalassemia minor (αα/- or α-/α-)
Three gene deletion resulting in HbH disease
Four gene deletion commonly called Hb
Bart’s hydrops fetalis
What are the classifications of B-Thalassemia? β°-Thalassemia (β-Thalassemia Major)
Sometimes called Cooley’s anemia
Β +
-Thalassemia (β-Thalassemia Intermediate)
β-Thalassemia (β-Thalassemia Minor)
Sometimes called β-thalassemia trait
What is the Hereditary Persistence of Fetal
Hemoglobin (HPFH)
Insufficient globin chain production.
Are structural hemoglobin variants arising from mutations in the globin genes and resulting in disruptions in the normal amino acid sequence in one or more of the globin chains of hemoglobin.
Used to describe a group of genetic conditions in which the concentration of HbF is increased above the reference interval with reduction β-globin synthesis and a compensatory increase in γ-globin synthesis.
What is Hemoglobinopathy?
What is Hemoglobinopaties?
Is a kind of genetic defect that results in abnormal structure of one of the globin chains of the hemoglobin molecule.
Are inherited single-gene disorders; in most
28
What is FBC?
What is FBE?
Chem PPT Flashcards, Unit 3
What is the Cyanmethemoglobin?
What is the Analytical Methodology for Hb
Determination of Cyanmethemoglobin?
What method is used for hemoglobin determination?
What is the completed blood count method?
What is the other method of hemoglobin determination?
What type of statins are used in hemoglobin determination?
How does the hemoglobin behave on agarose gel?
What is the advantage of using HPLC in hemoglobin determination?
What is the advantage of HPLC over electrophoresis on hemoglobin? cases, they are inherited as autosomal codominant traits. Common hemoglobinopathies include sickle-cell disease .
The oxidation of the Fe
2+
of hemoglobin to the Fe
3+
of methemoglobin by ferrycyanide, with methemoglobin then converted into stable cyanmethemoglobin by the addition of potassium cyanide (KCN).
Absorbance is measured at 540 nm and is used to calculate the concentration of hemoglobin.
Based upon the oxidation of the Fe 2+ of hemoglobin to the Fe
3+
of methemoglobin by ferrycyanide, with methemoglobin then converted into stable cyanmethemoglobin by the addition of potassium cyanide (KCN).
Completed Blood Count
It is a test that evaluates the cells that circulate in blood; also known as full blood count
(FBC), full blood exam (FBE), or blood panel.
It consists of counts of cells such as RBCs
(erythrocytes), WBCs (leukocytes), and platelets
A measure of hemoglobin; Estimates of the volume of red cells; and an estimation of white blood cells subtype (differential counting for neutrophils, lymphocytes, basophils, eosinophils, and monocytes).
Electrophoresis; Using agarose gel and a pH
9.2 barbital buffer
Stains would include Ponceau S (reddish staining), or preferably Amido black (dark blue to black staining)
Cannot accurately quantify HbA
2
, and comigration of many hemoglobin variants is observed
HPLC (High Performance Liquid
Chromatography); Uses a column packed with cation exchange resin to quantify HbA
2
and
HbF
Advantages over electrophoresis: superior resolution of hemoglobin variants rapid assay time accurate quantification of hemoglobin
29
Chem PPT Flashcards, Unit 3
How does the Capillary Electrophoresis being used in hemoglobin determination essay?
What is the use of Electrospray Mass
Spectrometry in hemoglobin variation?
What is the used of DNA analysis for hemoglobin:
What is the HbS Solubility Test?
When does the hemoglobin S produce visible turbidity?
List the substances used for determination of hemoglobin S in the sample?
How is HbS indicated in the sample?
What are the False-positive test results of HbS
Solubility?
How are the False-negative results of HbS obtained on samples?
What is the HbH test? fractions, including HbA
2
and HbF
Separation in an alkaline buffer using high voltages is based on (1) charge differences,
(2) electrolyte pH, and (3) electro-osmotic flow.
Has become the method of choice for the characterization of hemoglobin variants and hemoglobin adducts since it immediately establishes:
(1) whether the variant is an α or β-chain variant;
(2) the location and identity of the amino acid residue substitution;
(3) the quantity of variants present.
Diagnose and characterize α-thalassemia
Investigate potentially life-threatening disorders of hemoglobin synthesis in the fetus
Characterize the β-thalassemia genotype
Distinguish between conditions that have similar clinical and laboratory presentations but are due to different genetic conditions
Tests for specific hemoglobin variants; The most common type of abnormal hemoglobin and the basis of sickle cell trait and sickle cell anemia
HbS when oxygenated, is insoluble in concentrated phosphate buffer and produces visible turbidity, unlike the other hemoglobins
(A, F, C, E and D)
1) Using a reducing substance, sodium hydrosulfite (Na
2
S
2
O
4
, sodium dithionate);
2) Used to deoxygenate the hemoglobin and saponin to lyse the RBCs;
HbS is indicated by increased turbidity in the sample.
False-positive results are found in samples with Heinz bodies;
•
High concentrations of monoclonal protein or cold agglutinins.
False-negative results are obtained on anemic patients or on samples with hematocrit less than 15%.
Tests for specific hemoglobin variants;
It is a beta 4 (β4) insoluble tetramer moderate
30
What is Iron?
Chem PPT Flashcards, Unit 3
What are the charactistic of variant hemoglobins on HbH test?
How to detect unstable hemoglobins?
What is the importance of Iron in oxygen transport?
Where is iron distributed into?
What is/are stored iron form(s)?
What is/are transport iron form?
3+
What is apotransferin/Fe comple called?
What is the regulator of iron absorption?
What are common conditions decrease serum iron concentration?
What other conditions decrease serum iron to severe form of α-thalassemia characterized by pronounced microcytic hypochromic hemolytic anemia.
It punctuate inclusions, usually described as looking like "golf balls", are found in the RBCs of a peripheral blood smear that has been stained with new methylene blue or brilliant cresyl blue at 37°C. an increase in turbidity or complete precipitation in the blood sample was treated with heat at 55°C to 60°C or with isopropanol is used to detect the presence of unstable hemoglobins which precipitate in 3 to 4 minutes under this conditions. metal at the active site of many important redox enzymes dealing with cellular respiration and oxidation and reduction in plants and animal forms complexes with molecular oxygen in hemoglobin and myoglobin; these two compounds are common oxygen transport proteins in vertebrates
-
Hemoglobin
storage iron (ferritin and hemosiderin)
myoglobin
A labie iron pool
other tissue iron
transport iron (transferrin and apotransferrin)
ferritin
hemosiderin.
apotransferin .
T ransferrin
Hepcidin , A peptide hormone produced by the liver, is the central regulator of iron absorption
Children dietary deficiency because milk has low iron content and iron requirements for growth and development are high.
adults, iron deficiency is almost always the result of chronic blood loss or childbearing. patients with iron deficiency anemia and with chronic inflammatory disorders, such as (1) acute infection, (2) immunization, and (3) myocardial infarction.
blood donation
31
Chem PPT Flashcards, Unit 3 concentration?
Measurement of iron deficiency includes
What is IRON OVERLOAD?
What is the best term used to describe iron overload at tissue level?
In what condition, serum iron levels will be elevated?
What are the analytical methods for iron determination?
What are the Methods for the determination of the serum Ferritin?
What’s conditions known to affect serum iron concentration, TIBC and transferrin ssturarion
%?
What is Bilirubin?
hemorrhage
menstruation.
-
Serum iron determination
Iron binding capacity
-
Serum ferritin
-
Stainable iron in the bone marrow
-
Erythrocyte protoporphyrin
Circulating transferrin receptor and reticulocyte hemoglobin
DNA analyses
Hemochromatosis and types of anemia associated with ineffective erythropoiesis.
Hemosiderosis
siderosis
patients with aplastic anemia
children with acute iron poisoning
after oral or parenteral iron use
acute liver injury
The use of hormonal contraceptive
-
Colorimetric method
Transferrin Saturation %
-
TIBC = UIBC + serum iron
-
Serum transferrin (g/L) = 0.007 x
TIBC (ug/dL)
-
Immunoradiometric assay
-
Enzyme-linked Immunosorbent assay
(ELISA)
Immunochemiluminescence assays
-
Immunofluorometric methods
Diurnal variation
Menstrual cycle
Pregnancy
Ingestion of iron
Oral contraceptives
Iron dextran injections
Hepatitis
Acute inflammation
Iron deficiency
Iron overload
Bilirubin is the orange yellow pigment derived mainly from aging red blood cells that are destroyed in the reticuloendothelial cells of the liver, spleen and bone morrow.
Where is Bilirubin extracted and metabolized? In the liver.
Where is Bilirubin excreted? In bile and in the urine.
What is Bilirubin the end product of? Hemoglobin metabolism.
32
Bilirubin
Metabolism
Chem PPT Flashcards, Unit 3
Continuation of Bilirubin
Metabolism
Continuation of Bilirubin
Metabolism
Continuation of Bilirubin
Metabolism
-
Bilirubin (B1)
Attaches to
albumin
Liver
UDP-glucoronyl transferase
(uridine diphosphate)
Bilirubin monoglucoronide
-
Bilirubin monoglucoronide
UDP-glucoronyl transferase
(uridine diphosphate)
Bilirubin diglucoronide (B2)
Bile
Intestines
Intestines (normal flora)
Urobilinogen
33
Continuation of Bilirubin
Metabolism
Chem PPT Flashcards, Unit 3
What are other names of Bilirubin 1?
What are other names of Bilirubin 2?
What are some clinical significance of
Bilirubin?
How does someone with Jaundice or hyperbilirubinemia looks like?
What is another name for this?
Oxidation
Unchanged
Stercobilinogen
Urobilins
(stool)
Stercobilin
(stool)
Urobilin
(urine)
Reabsorb by the enterohepatic circulation
(Enterohepatic cycle)
1) Unconjugated bilirubin
2) Water insoluble / Non-polar bilirubin
3) Indirect reacting bilirubin
4) Hemobilirubin
5) Free bilirubin / Unbound bilirubin
6) Prehepatic bilirubin
!) Conjugated bilirubin
2) Water soluble / Polar bilirubin
3) Direct reacting bilirubin
4) Cholebilirubin / cholestatic bilirubin
5) One-minute bilirubin / Prompt bilirubin
6) Post hepatic bilirubin
7) Obstructive bilirubin
8) Regurgitative bilirubin
Jaundice or hyperbilirubinemia
They have yellow discoloration or pigmentation of the skin, sclera and mucous membranes.
Also called Icterus
34
Chem PPT Flashcards, Unit 3
When does hyperbilirubinemi~a becomes clinically evident?
What are some classifications of Jaundice?
When serum bilirubin levels exceed 2.5 mg/dL (normal 0.3-1.0 mg/dL)
1) Increased serum unconjugated bilirubin.
2) Result of excessive bilirubin presented to the liver.
The classifications of Jaundice are also seen in? 1) HDN (hemolytic disease of the newborn)
2) Malaria
3) Extensive hematoma
4) Hemolytic transfusion reaction
Post- Hepatic jaundice increased ____ bilirubin High levers of unconjugated
Kernicterus refers to ?
Kernicterus is commoly seen in ?
The yellow staining caused by bilirubin
Newborns
If levels of bilirubin are very high the substance will move out of the blood and collect in
Brain tissue
What are some of the symtoms of kernicterus? Yellow of the eyes
Excess bilirubin in the blood
Treatment of kernicterus ?
Jaundice types?
Light therapy
Exchange transfusions
Pre-hepatic (hemolytic)
Hepatic (hepatocellular)
Post-hepatic (obstructive)
Hemolytic anemia can cause ____ Jaundice
True or false failure of bile to flow to the intestines due to an obstruction in the biliary tree
Where has post hepatic seen in ?
Pre-hepatic
True
Caused of hepatic jaundice ?
Clinical examples of intrahepatic jaundice cause by genetic errors in bilirubin metabolism
Prehepatic jaundice caused by hemolysis
What are the two inherited disorders in which bilirubin is conjugated ?
What is the origin of Physiologic Neonatal
Jaundice?
Choledocholelithiasis
Biliary atresia
Parasitism
By increase of both unconjugated and conjugated bilirubin levels
Gilberts syndrome, dubin –johnson and rotor syndronme
Autoimmune abnormal Hb
Intrahepatic jaundice caused by infection? Hep A,B,C
Clinical examof intrahepatic jaundice seen in Physiologic neonates
What is lucey driscoll syndrome ? Familial form of unconjugated hyperbilirubinemia caused by circulating
How long does hyperbilirubinemia lasts?
Difference between type 1 and type 11 criger najjar syndrome ? inhibitor of bilirubin conjugation
2 to 3 week’s of life
Type 11 is less severe with a response to phenobarbital p450 inducer
Dubin Johnson syndrome, rotor syndrome
Hepatic
35
Chem PPT Flashcards, Unit 3
Deficiency of which enzyme leads to
Physiologic Neonatal Jaundice (Physiological
Jaundice of the Newborn)?
What are the factors contributing to physiological jaundice?
How is the physiological jaundice of the newborn is treated?
How bilirubin is measured in body fluids? uridine diphosphate glucuronyl transferase
(not fully developed)
- Increased bilirubin load in the newborn
-Decrease conjugation of bilirubin resulting from relative lack of UDPGT enzyme
-Exposure of breast-feeding infants to pregnanediol, nonesterified fatty acids, and other inhibitors of bilirubin conjugation
It is treated with phototherapy; the infant is exposed to light of approximately 450nm that disrupts intramolecular hydrogen bonds in the bilirubin molecule and yields photoisomers that are water-soluble and thus are excreted in the bile.
1. Spectrophotometric (Diazo - Chemical,
Direc Spectrophotometric, Enzymatic, and
Transcutaneous) methods
2. Chromatographic methods
Methanol
Which substance acts as coupling accelerator in
Evelyn and Malloy?
Which substance acts as coupling accelerator in
Doumas and colleagues?
Van den Bergh and Muller method.
Total Bilirubin method.
Direct Bilirubin method.
What s ditaurobilirubin?
Application of High-Performance Liquid
Chromatography (HPLC)
Enzymatic methods for bilirubin determination.
Sodium Benzoate
Involves the coupling of bilirubin with diazotized sulfanilic acid (Ehrlich diazo reagent)
Serum is added to an aqueous solution of caffeine and sodium benzoate, and after 10 minute incubation at room temperature alkaline tartrate is added.
Bilirubin monoconjugates and di-conjugates
(mainly glucuronides) and δ- bilirubin, because they are water-soluble, react with the diazo reagents in the absence of accelerator.
It is a water-soluble synthetic material, is used by instrument manufacturers for calibrating direct bilirubin methods; it is also present in materials used for quality control and for proficiency testing.
It is used for rapid separation and quantification of 4 main bilirubin fractions:
(1) α-unconjugated bilirubin; (2) β-bilirubin monoglucuronide; (3) γ-bilirubin diglucuronide; and (4) δ-bilirubin
At the pH near 8 and in the presence of sodium cholate and sodium dodecylsulfate, on
36
Chem PPT Flashcards, Unit 3 four bilirubin fractions are oxidized to purple and finally colorless products.
The decrease in absorbance at 425 or 460nm is proportional to the concentration of total bilirubin
Conjugated Bilrubin (B2)
Which form of bilirubin is excreted in urine and its presence indicates conjugated hyperbilirubinemia.?
What is Porphyrins?
What is the parent porphyrin?
What are the functions of porphyrins?
What does ALA stands for?
What does PBGstands for?
Which are the precursors of porphyrin?
Which are the enzymes used for the Heme biosynthesis?
What are functions of Heme?
Porphyrins are a group of heterocyclic macrocycle organic compounds, composed of four modified pyrrole subunits interconnected at their α-carbon atoms via methene bridges.
The parent porphyrin is porphin
Porphyrins are essential for the function of hemoglobin — a protein in red blood cells that links to porphyrin, binds iron, and carries oxygen to different organs and tissue.
Aminolevulinic acid
Porphobilinogen
Aminolevulinic acid (ALA) and
Solubility of porphyrin precursors.
Porphobilinogen (PBG) porphyrin precursors that are highly water soluble.
Which is the major component of hemoglobin? Heme is a major component of hemoglobin,
Which tissue organ made Heme?
Heme is made mainly in the bone marrow and liver through the production of porphyrin and linkage with iron.
What is the main function of Heme? The protein in red blood cells that carries oxygen from the lungs to all parts of your body.
5-Aminolevulinate Synthase, ALAS
5-Aminolevulinic Acid Dehydratase, ALAD
Hydroxymethylbilane Synthase, HMBS
Uroporphyrinogen-III Synthase, UROS
Uroporphyrinogen Decarboxylase, UROD
Coproporphyrinogen Oxidase, CPOX
Protoporphyrinogen Oxidase, PPOX
Ferrochelatase, FECH
Heme containing proteins participate in a variety of redox reactions:
-Oxygen transport (by hemoglobin in the blood) and storage (by myoglobin in muscle)
-Mitochondrial respiration
-Enzymatic destruction of peroxides (by catalase and peroxidase)
37
Chem PPT Flashcards, Unit 3
Which are the Precursors Excretion of Heme in urine?
Which are the Precursors Excretion of Heme in feces?
What is the Porphyria?
Which organs are affected by the Porphyria?
-Drug metabolism
-Desaturation of fatty acids
-Tryptophan metabolism (by tryptophan oxygenase
Aminolevulinic acid (ALA), porphobilinogen
(PBG), uroporphyrinogen, and coproporphyrinogen III are excreted in urine
Protoporphyrin, protoporphyrinogen and coproporphyrinogen-I are excreted in feces
Porphyria refers to a group of disorders that result from a buildup of natural chemicals that produce porphyrin in the body.
Porphyria mainly affects your nervous system, skin and other organs. The signs and symptoms of porphyria can vary, depending on the specific type and severity.
Types of porphyria.
Which are the drugs cause acute porphyrias?
-porphyria — acute , which mainly affects the nervous system,
- nonacute or cutaneous , which mainly affects the skin.
Acute porphyrias include forms of the
Types of Acute pophyrias. disease that typically cause nervous system symptoms, which appear quickly and can be life-threatening.
Acute intermittent porphyria (AIP)
Variegate porphyria (VP)
Hereditary coproporphyria (HCP)
Which are the factors cause acute porphyrias?
The most important precipitating factors are
(1) drugs, (2) alcohol, especially binge drinking, (3) the menstrual cycle, (4) calorie restriction, (5) infection, and (6) stress.
Drugs known to provoke acute attacks include
(1) barbiturates, (2) sulfonamides, (3) progestogens, and (4) many anticonvulsants.
Which are the Possible signs and symptoms of -Severe abdominal pain acute porphyria? -Swelling of the abdomen (abdominal distention)
-Pain in your chest, legs or back
-Constipation or diarrhea
-Vomiting
-Insomnia
-Heartbeat (palpitations)
ACUTE PORPHYRIA
The following symptoms of High blood pressure, Anxiety or restlessness, Seizures,
Mental changes, such as confusion, hallucinations, disorientation or paranoia,
38
Chem PPT Flashcards, Unit 3
Breathing problems, Muscle pain, tingling, numbness, weakness or paralysis, Red or brown urine are associated with what?
What type of porphyria(s) include forms of the disease that cause skin symptoms as a result of oversensitivity to sunlight, but does don't usually affect the nervous system?
What type of porphyria have attacks that last for several days, with some forms, signs and symptoms that may start during infancy or childhood?
What are the two categories of nonacute or cutaneous porphyrias?
Cutaneous or nonacute porphyrias
CUTANEOUS PORPHYRIAS
Name 1 type of symptom of CUTANEOUS
PORPHYRIAS As a result of sun exposure.
Exposure to various toxins such as lead can cause what effects?
The definitive test is used for lead toxicity and measures what?
Secondary coproporphyrinuria is also caused by what?
What is Hereditary tyrosinemia type I?
What is going on physiological during Renal disorders?
Hepatobiliary disorders cause what?
1) Bullous skin lesions:
Porphyria Cutanea Tarda (PCT)
Congenital Erythropoietic Porphyria (CEP)
2) Acute photosensitivity:
Erythropoietic protoporphyria (EPP)
X-linked dominant protoporphyria (XLDPP)
Sensitivity to the sun and sometimes artificial light, causing burning pain
Sudden painful skin redness
(erythema) and swelling (edema); blisters that take weeks to heal
Itching, fragile skin
Scars or skin color changes from healing blisters
Increased hair growth
Red or brown urine
Increases the urinary ALA and coproporphyrin-III excretion and cause accumulation of zinc protoporphyrin (ZPP) in erythrocytes.
Measurement of blood lead and ZPP measurements
Toxic effects of alcohol, arsenic, other heavy metals and various drugs.
Succinylacetone, which accumulates in this disease, has a structural resemblance to ALA and therefore a competitive inhibitor of
ALAD.
Patients suffer neurological crisis very similar to attacks of acute porphyria.
Impaired glomerular function reduces the clearance of water-soluble porphyrins normal excreted in the urine
Urinary excretion of coproporphyrin-I is
39
Chem PPT Flashcards, Unit 3
Give an example a Hematological disorders
Dietary, bacterial, and gastrointestinal bleeding factors cause what?
What is Pseudoporphyria?
There are 8 different Laboratory Diagnosis for
Porphyria, name 1. increased in Dubin-Johnson syndrome, Rotor syndrome and in Gilbert's disease.
1)In iron deficiency anemia, zinc acts as an alternative substrate for ferrochelatase
(FECH, also known as heme synthase), leading to increased ZPP.
2)Increased red cell protoporphyrin (mostly
ZPP) may also occur in sideroblastic, megaloblastic and hemolytic anemia
Porphyrins may also come directly from the diet
additional protoporphyrin and other dicarboxylic porphyrins may be formed by the action of gut flora on heme-containing proteins derived from the diet or by gastrointestinal hemorrhage.
Patients with PCT likely skin lesions with no accumulation of porphyrin's demonstrated
1.Urinary porphobilinogen (PBG) determination
2.Fecal and Urine Porphyrin determination
3.High Performance Liquid Chromatography
(HPLC)
4.Fluorescence Emission Spectroscopy
5.Erythrocyte/Whole blood Porphyrin measurement
6.Analysis of Plasma Porphyrins
7.Enzyme measurements
8.DNA analysis light All samples for porphyrin assay must be protected from what?
Urinary concentrations decrease by up to percentage if exposed to light for 24 hours
Porphyrins and PBG are best analyzed in fresh, early-morning (10 to 20 mL) specimens collected without preservative. They are stable in urine in the dark at 4°C for up to how many hours and for at least a month at -20°C.
Specimens for ALA should be properly refrigerated. Urine specimens can be stored at
4°C in the dark for at least ? weeks without significant loss of ALA, and frozen specimens are stable for weeks.
PBG is more stable around pH ?, ALA is more stable around pH ? , although more acidic environments greatly reduced ALA stability.
50%
48 hours
2 weeks
PBG pH of 8 to 9
ALA pH of 3 to 4
40
Chem PPT Flashcards, Unit 3
How many grams wet weight of feces is adequate for porphyrin measurements stable for many months at what temperature?
EDTA whole blood shows no loss of protoporphyrin for how many days at room temperature? and for at least eight weeks at 4°C in the dark
EDTA whole blood shows no loss of protoporphyrin for how many weeks being refrigerated in the dark?
All samples received from patients with suspected bullous porphyria are treated as what and why?
What are 4 methods that are used for searching for metabolites of Porphobilinogen (PBG)?
What color does Watson-Schwartz and Hoesch methods stain Porphobilinogen?
How is 5-Aminolevulinic acid (ALA) usually converted into an Ehrlich-reacting pyrrole?
What is this method known as?
What does the spectrophotometric scanning of acidified of porphyrins in urine or fecal extracts for the presence of?
Name 3 other methods that are used to look for porphyrin in Urine and Feces.
5 to 10 and -20°C
8 days
8 weeks
"High risk" because the frequency of infection with hepatotrophic viruses, particularly HCV, is increased in PCT
Watson-Schwartz and Hoesch methods
High performance liquid chromatography
(HPLC)
Ion Exchange Chromatography
Tandem Mass Spectrometry
•
Rose-red or Magenta
By condensation with a reagent such as
• acetylacetone, after separation from PBG.
Mauzerall and Granick method
Soret band
What is the current method of choice for looking for porphyrin in Urine and Feces?
What are the 3 methods used to look for blood porphyrins?
What are the 2 methods used to look for plasma porphyrins ?
Enzyme measurements are?
DNA analysis is?
DNA analysis is used to?
What is Therapeutic Drug
Monitoring/Management (TDM)?
Therapeutic Drug Monitoring/Management
Fluorometric methods
Paper and Thin Layer Chromatography
Reversed-phase High Performance Liquid
Chromatography (HPLC) a.
Reversed-phase High Performance Liquid
Chromatography (HPLC)
Piomelli method
Blake et al method
Spectrofluorometric method
Fluorescence Emission Spectroscopy
HPLC
Rarely required, but useful for family studies and for identification of subtypes.
Identification of mutation from family member with definite diagnosis.
Screen of relatives for mutation
A process used to measure blood drug levels so that the most effective dosage is maintained and toxicity prevented.
Drug dosages
41
Chem PPT Flashcards, Unit 3
(TDM) is a multidisciplinary science of individualization of what?
What does Therapeutic Drug
Monitoring/Management (TDM) do to synthesize clinical information and laboratory testing results?
Therapeutic drug monitoring allows assessment of what?
What does therapeutic drug monitoring indicate?
What is pharmacogenomics?
To facilitate selection of the optimal drug and dose for each patient
Therapeutic compliance and efficacy
Detection of drug interactions
Drug-induced toxicity
How much of the drug has been absorbed, distributed, metabolized, and eliminated.
What is pharmacodynamics (PD)?
What is another statement used for pharmacodynamics?
The pharmacologically active substance produces an effect on a living organism or in a biochemical system is known as?
What is the site of action?
The study of the inherited variations in genes that dictate drug response and the way these can be used to predict individual responses to a drug, using a genome-wide approach.
The study of the physiological response to drugs which encompasses the interaction of drugs with target sites, and the biochemical and physiological consequences that lead to therapeutic or adverse effect
“What the drug does to the body?”
Mechanism of action
The location (organ or specific cell type) of the target molecules upon which a drug acts
Enzyme or a transporter Most drugs exert their effects by binding to a protein target such as?
Many drugs have increasing effects with ____ dose.
Explain some of a therapeutic drug’s mechanism of action
Increasing
What is a Drug biologic half-life or terminal half-life?
Therapeutic drugs mechanism of action include:
Therapeutic Range:
•Represents the relationship between minimum effective concentration (MEC) and minimum toxic concentration (MTC).
Trough concentration:
•The lowest concentration achieved just before the next dose
Peak concentration:
•The highest concentration achieved within the dosing cycle.
The time it takes for a drug to lose half of its pharmacologic, physiologic, or radiologic
42
Chem PPT Flashcards, Unit 3
What is xenobiotics?
Define Pharmacokinetics (PK) activity.
This means that it takes 4 to 5 times the halflife for a drug's serum concentration to reach steady state after regular dosing is started, stopped, or the dose changed.
A chemical compound that is foreign to a living organism
Pharmacokinetics (PK) is Øthe mathematical description of the physiological disposition of xenobiotics or endogenous chemicals,
“what the body does to a drug”
What is ADME ?
How can pharmacokinetic absorption be describe?
The processes of A bsorption, D istribution,
M etabolism, and E limination included in the activity or fate of drugs in the body over a period of time. These processes are affected by factors specific to the individual receiving the drug including disease state, comedication, age and sex.
Oral dosing requires the drug to pass from the
GIT into the vascular system through a process known as absorption .
To be absorbed, the drug has to be dissociated
(called liberation ) then must cross through cell membranes by passive diffusion.
Intravenous (IV) delivery What is the most direct route of administering a drug?
What is a drug’s bioavailability?
How does a drug distribution work?
Bioavailability is the amount of drug absorbed relative to the quantity given.
Bioavailability is affected by first-pass metabolism (intestines and liver) which will reduce the quantity of drug to reach the systemic circulation.
Drugs undergo distribution once in the bloodstream, and spreads throughout the systemic circulation and into various tissues.
What does distribution of a drug depends on? The distribution of a drug to a particular site depends on (1) molecular size, (2) degree of ionization, (3) lipid solubility, (4) extent of protein binding and (5) body composition .
Drugs that distribute extensively into tissues tend to be lipophilic, as this facilitates passage through cell membranes .
What are some of the plasma protein drugs bind to?
Albumin, globulins, and lipoproteins.
Which protein do acidic or basic drugs bind to? Acidic drugs associate primarily with albumin
Basic drugs preferentially bind globulins and
43
Chem PPT Flashcards, Unit 3 lipoproteins
What is a Pharmacokinetic drug metabolism? It is the biotransformation of a compound, whether endogenous or exogenous.
Drug metabolism is typically the result of
What is elimination? enzymatic activity, found in the liver, GIT and kidneys.
The final removal of drugs from the body
What are the steps in elimination?
List some of the clinical utility of Therapeutic
Drug Monitoring/Management (TDM)
Most common routes are excretion into urine or stool. Drugs are eliminated into breast milk, sweat and hair.
Clearance can be measured directly and renal elimination can be estimated by using the glomerular filtration rate.
In practice, urine is rarely used for TDM purposes
TDM can be used to assess compliance, address physiological or pathological changes, and maintain optimal dosing for each individual patient.
It is also useful in the management of many conditions requiring long-term pharmacological therapy like hyperlipidemia and hypertension.
TDM can also guide initial selection and dosing of a drug.Routine TDM is also helpful for detecting and managing alterations in drug dispositions within an individual.
The potential to detect noncompliance What is a major asset of consistent use of
TDM?
What are some concerns in Therapeutic Drug
Monitoring (TDM)?
What does GC-MS stand for?
What does LC-MS/MS stand for?
What does HPLC-UV stand for?
Name 6 traditional antiepileptics?
The need for accurate, reproducible methods
Requirement for quality assurance and proficiency testing programs
Necessity for establishing target ranges
Gas Chromatography-Mass Spectrometry
Liquid Chromatography-Mass
Spectrometry/Mass Spectrometry
High Performance Liquid Chromatography-
Ultraviolet
Benzodiazepines
Carbamazepine
Ethosuximide
Phenobarbital and Primidone
Phenytoin and Fosphenytoin
Valproic acid
44
Chem PPT Flashcards, Unit 3
What are the effects of Benzodiazepines?
Benzodiazepines work by?
sedative, hypnotic (sleep-inducing), anxiolytic (anti-anxiety), anticonvulsant, and muscle relaxant properties.
Enhancing the effect of the neurotransmitter gamma-aminobutyric acid (GABA) at the
GABA
A
receptor.
Diazapam (Valium) and Clonazepam
15 mg/L
What are 2 kinds of benzodiazepines?
Carbamazepine toxicity is associated if levels exceed?
What is the mode of action of Carbamazepine and what are its effects?
Ethosuximide (Zarontin) is used for the treatment of?
How does Ethosuximide take effect in the body?
Phenobarbital (Luminal) and Primidone
(Mysoline) affect the body in what manner?
Phenytoin (Diphenylhydantoin, Dilantin) and
Fosphenytoin (Cerebyx) are used in the treatment of?
What is the mode of action and the effects of phenytoin and fosphenytoin?
Modulates the synaptic sodium channel, which acts to reduce central synaptic transmission, aiding in control of abnormal neuronal excitability
Absence seizures characterized by brief loss of consciousness.
It reduces the flow of calcium through T-type calcium channels in the synapse and slows the rate of this seizure inducing pulses.
By reducing synaptic transmission through action on the GABA
A
receptor, resulting in decreased neuronal excitability.
Used in the treatment of all but absent seizures
They interfere with sodium channel activity by prolonging inactivation, which reduces synaptic transmission and assist in control of abnormal neuronal excitability. absence seizures Valproic acid (Depakote) is most commonly used for treatment of?
What is the mode of action and the effects of
Valproic acid?
Hepatic toxicity and acute toxic encephalopathy have been associated with what concentration of valproic acid?
Name 6 contemporary antiepileptics:
Which contemporary antiepileptic promotes the release of GABA but does not interact directly with the GABA receptor?
It inhibits the enzyme GABA transaminase, resulting in increased concentrations of
GABA and overall inhibition of neuronal activity in the brain.
Concentrations greater than 100 mg/L
Gabapentin
Lamotrigine
Levetiracetam
Oxcarbazepine
Topiramate
Zonisamide
Gabapentin (Neurontin)
45
Chem PPT Flashcards, Unit 3
What is Lamotrigine and how does it affect the body?
Name the broad spectrum antiepileptic that acts through synaptic vesicle protein SV2A, which is involved in the release of neurotransmitters from presynaptic terminals?
What antiepileptic drug is metabolized to monohydroxycarbamazepine (MHC), the metabolites responsible for the therapeutic effect?
Which broad spectrum antiepileptic drug that has sodium and calcium channel blocking activity, potentiates the activity of GABA, and inhibits glutamate release?
What is Zonisamide (Zonegran)?
Antifungal or Antibacterial agents?
1) Aminoglycosides
2) Triazoles
3) Vancomycin
4) Amikacin
5) Gentamicin
6) Tobramycin
How does bacterial susceptibility to antibiotics being measured?
A broad spectrum antiepileptic drug that acts through multiple mechanism including blocking sodium and calcium channels and reducing glutamate release
Levetiracetam (Keppra)
Oxcarbazepine (Trileptal)
Topiramate (Topomax)
Is a sodium and calcium channel blocker and considered a broad spectrum antiepileptic
1) Antibacterial
2) Antifungal
3) Antibacterial
4) Antibacterial
5) Antibacterial
6) Antibacterial
What is minimum inhibitory concentration mean?
Gentamicin is an example of Aminoglycosides which inhibits what?
What do you call a glycopeptide antibiotic with activity against antibiotic-resistant bacteria, including methicillin-resistant Staphylococcus aureus (MRSA)?
What most common pathogen species are being inhibited by antifungal agents?
Voriconazole (Vfend) and Posaconazole (
Noxafil) are examples of what type of antifungal agent?
__________ is an antifungal agent that has a broad-spectrum compounds that kills by inhibiting synthesis of the major fungal sterol,
Bacterial susceptibility to antibiotics is commonly measured in terms of the minimum inhibitory concentration (MIC), the concentration of drug sufficient to inhibit growth of an organism.
The concentration of drug sufficient to inhibit growth of an organism.
They inhibit protein synthesis to kill aerobic, gram-negative bacteria
Vancomycin (Vancocin)
The most common pathogens are species of
Candida yeast or Aspergillus molds
Triazole
Posaconazole ( Noxafil)
46
Chem PPT Flashcards, Unit 3 ergosterol.
What do you call a chemotherapeutic drug that inhibits the growth of malignant cells by alkylating DNA?
What antitumor agent can also minimize the risk of secondary tumor development and growth retardation in children when compared with irradiation?
Identify to which immunosuppressant they belong to:
1) Cyclosporine
2) Tacrolimus
3) Mycophenolate Mofetil
4) Sirolimus
5) Everolimus
What do you call the drugs capable of suppressing immune responses used to treat autoimmune diseases, allergies, multiple myeloma, other cancers and chronic nephritis, and most important, to prevent rejection in
Busulfan
Busulfan
What anti-neoplastic s or anti-cancer drugs is being used in the management of acute lymphoblastic leukemia, choriocarcinoma, elated trophoblastic tumor, and in maintenance of remission in leukemia and treatment of severe psoriasis?
_____________ inhibits DNA synthesis and competitively inhibits the enzyme dihydrofolate reductase.
What are arrhythmias?
Methotrexate
Methotrexate
Arrhythmias are disturbances in normal cardiac sinus rhythm and are sometimes associated with substantial morbidity and mortality.
What is the most common serious arrhythmia? Atrial fibrillation
Many antiarrhythmic drugs exert their action by Na+, K+ or Ca2+ regulation of what cation channels?
What cardioactive drug is obtained from Digoxin (Lanoxin)
Digitalis plants such as the foxglove, it is a cardiac glycoside used in the treatment of arrhythmias and heart failure?
What does Digoxin (Lanoxin) do to your heart rate?
It acts by slowing heart rate, increases the strength and velocity of cardiac contraction and regulate the nervous (sympathetic) and endocrine (renin-angiotensin) system.
What cardioactive drug is particularly prescribed in cases of congestive heart failure?
Digoxin (Lanoxin)
1) Calcineurin inhibitor
2) Calcineurin inhibitor
3) IMPDH inhibitor
4) mTOR inhibitor
5) mTOR inhibitor
Immunosuppressant drugs
47
Chem PPT Flashcards, Unit 3 organ or bone marrow transplantation?
_________is important in optimizing immunosuppressant therapy because of possible serious consequences of under dosing or overdosing.
What prevents drug-related toxicity and is used to evaluate compliance?
Cyclosporine is a immunosuppressant derived from bacterial sources. True or
False
TDM (Therapeutic drug monitoring)
TDM (Therapeutic drug monitoring)
False; it comes from Fungus
Cyclosporine is a fat______ cyclical peptide that______ the activation of T lymphocytes via a multifaceted mechanism.
A soluble, blocks
B insoluble, enables
C neutral, inhibits
D rich, starts
Tacrolimus has two names. Identify them.
A.
Prograf
B.
FK506
C.
BGM109
TLM1
A
A and B
Cellcept arrests T-cell proliferation by the suppression of guanine nucleotide production when IMPDH is inhibited by
MPA. True or False
A.
True
B. False
T
Opioids can be monitored in urine to detect compliance, diversion, and use of nonperscribed opioids?
A.
True
False
Codeine is a fully synthetic opioid. True or
False?
A.
True
A
A.
B
False
Which one of these choices is a nueraleptic agent?
A.
Lithium
B.
Classical Antipsychotics
C.
A and B
C
None of the above
Toxicology is most specifically a branch of ___ Pharmacology
Toxicology is concerned with the study of Living organisms
48
Chem PPT Flashcards, Unit 3 adverse effects of chemicals on ___.
There are 9 different procedures for detection of drugs. Name as many as you can:
Spot test, determination of anion gap, electrocardiogram, determination of Osmol gap, immunoassay, planar chromatography, gas chromatography, high performance liquid chromatography, point of care devices qualitative Spot tests are qualitative or quantitative?
Do Spot tests suggest or prove: Suggest
Name two examples of spot tests for salicylate: Ferric chloride test, Trinder test
What is the formula used to identify increased anion gap?
AG = [Na+] - [Cl- + HCO3-]
What is the reference interval for anion gap determination?
What are the common causes of anion gap?
What is the MUDPILES mnemonic?
8 to 16 mmol/L
Name the causes of persistent anion gap.
Correct interpretation of results facilitates:
MUDPILES mnemonic
Methanol
Uremia
Diabetic ketoacidosis
Paraldehyde
Iron, inhalants, isoniazid, ibuprofen
Lactic acidosis
Ethylene glycol, ethanol ketoacidosis
Salicylates, starvation ketoacidosis, sympathomimetics
Continued absorption of exogenous acids
Formation of acidic metabolites
Cellular ischemia with worsening lactic acidosis
Laboratory testing
Diagnosis of poisoning
Management
Electrocardiogram (ECG) What is one of the screening procedure for detection of drugs
What are the formulas to determine Osmol
Gap?
What are the formulas to determine Osmol
Gap?
How do you determine the Osmol Gap
OSMc (mOsm/kg) = 2Na (mmol/L) + glucose
(mg/dL)/18 + urea (mg/dL)/2.8
OSMc (mOsm/kg) = 2Na (mmol/L) + glucose
(mmol/L) + urea (mmol/L)
OSMc (mOsm/kg) = 1.86 Na (mmol/L) + glucose (mg/dL)/18 + urea (mg/dL)/2.8 + 9
OSMc (mOsm/kg) = 1.86 Na (mmol/L) + glucose (mmol/L) + urea (mmol/L)+ 9
OSMg = OSMm – OSMc
Difference between Actual osmolality
(measured) and the calculated osmolality
Immunoassay What is the method of choice for initial screening of most drugs of abuse
True or False True
49
Chem PPT Flashcards, Unit 3
Immunoassay tests for drugs of abuse are capable of semiquantitative results.
Planar Chromatography is also referred to as.. Thin layer chromatography
Specimens for planar chromatography to Urine, serum, gastric contents. determine drugs of abuse include?
What is the specimen of choice for planar chromatography and why?
Urine, because most drugs and drug metabolites are present in urine in relatively high concentrations.
Gas Chromatography What is one type of screening procedure for the detection of drugs?
What else is gas chromatography known as? Gas liquid chromatography (GLC, GC)
It is rapid, and capable of resolving broadspectrum of drugs
Qualitative and quantitative
Why is gas chromatography used in drug screening?
It is widely used for what two kinds of drug analysis?
How does gas chromatography analyze specimens? What are some parts of the procedure?
What procedure achieves the greatest accuracy in drug screening?
What procedure is used for comprehensive drug screening in biological fluids?
What are some advantages HPLC has over gas chromatography?
It uses capillary column, flame ionization and alkali flame ionization for analysis
Gas chromatography coupled to a mass spectrometer (GC-MS)
High-Performance liquid chromatography
(HPLC)
The incorporation of a diode array detector has what effect?
What are some advantages of Point-of-care devices?
1) It can analyze polar compounds without derivatization
It can analyze thermally labile drugs
It greatly increases the discriminatory power of this technique
1) Easy to use
2) durable
3) portable
4) rapid may be adjusted to detect one or many drugs
Urine and Saliva What body fluids are used in these types of point of car devices?
Carbon monoxide (CO) are agents that cause
____ _____.
You are considered legally intoxicated at
0.08% if you consume what?
Cellular Hypoxia
Alcohol
Bought over the counter and can cause hepatic and renal toxicity with overdose.
Acetylcholine is what type of agent
Any drug that is repeatedly and deliberately used in a way other than prescribed or socially sanctioned is considered what?
DFSA is abbreviation for?
Non-prescription Analgesics
Cholinergic
Drug Abuse
Drug Facilitated Sexual Assault
50
Chem PPT Flashcards, Unit 3
What does CO stand for?
How can CO be described?
What is CO the product of?
Carbon Monoxide
Colorless, odorless, and tasteless
Incomplete combustion of carbonaceous material
CO will combine readily to what?
What is the produce when CO binds with Heme
Heme Fe2+ of hemoglobin
Carboxyhemoglobin
Fe2+?
True or False
The binding affinity of hemoglobin for CO is about 250 times weaker than that for oxygen
False. The binding affinity for hemoglobin and CO is 250 times greater than that for oxygen
What will CO compete with for hemoglobin? Oxygen
CO will decrease what content?
Blood oxygen content and oxygen’s availability to tissue
What is the treatment for the above situation? Remove individual from contaminated area and administer oxygen
How can CO be measured in the blood?
Which method of measurement for CO is described:
Accurate, precise, and considered to be the reference procedure
Which method of measurement for CO is described:
Fast, convenient, accurate, precise
Gas chromatography and spectrophotometry
Gas chromatography
Spectrophotometry
What will Cyanide bind to? Heme iron in the cytochrome within mitochondria and cross biological membranes
What are symptoms of rapid onset of hypoxia? Flushing, headaches, tachypnea, dizziness, and respiratory depression
What is tachypnea?
What can respiratory depression lead to?
Abnormal rapid breathing
Coma, seizures, complete heart block, and death
What is the treatment for cyanide?
How is methemoglobin formed?
Hydroxycobalamin, or the cyanide antidote kit
When the heme iron in hemoglobin (Fe2+) is oxidized to the Fe3+ state
Does methemoglobin bind to oxygen? no
What is the enzyme responsible for maintaining hemoglobin iron in the reduced state? nicotinamide adenine dinucleotide (NADH)methemoglobin reductase in congenital methemoglobinemia Where is the deficiency of the enzyme is seen?
Acquired toxic methemoglobinemia may be caused by what?
A normal pO2 in a cyanotic patient indicates what?
Specific therapy for toxic methemoglobinemia involves what?
Methemoglobinemia is measured in various drugs and chemicals. is a significant indication of the possible presence of methemoglobinemia. the administration of methylene blue. taking automated multi-wavelength measurements
51
Chem PPT Flashcards, Unit 3 blood manually or by using what automated method? with a co-oximeter.
Where should keep Methemoglobin-forming
Agent?
Since methemoglobin is not stable at room temperature, specimens should be kept on ice or refrigerated but not frozen (freezing results in an increase in methemoglobin concentration).
What is toxic that most widely used and often abused chemical substances?
Where do the actions of ethanol involve?
Ethanol is one of several alcohols that is toxic and medically important.
How is the blood alcohol concentration that is allowed by DMV?
Central nervous system (CNS) depressant
You are considered legally intoxicated at a
BAC of 0.08% (80 mg/dL)
How long is take for BAC level to release alcohol?
Where does Ethanol metobolize?
What is the side effect of Ethanol?
How can we analyze Ethanol In blood?
How can we analyze Ethanol In blood?
It can take up to 6 hours for BAC level to drop from a 0.08 to a 0.00
Ethanol is metabolism by the liver alcohol dehydrogenase to acetaldehyde, which is subsequently oxidized to acetic acid by aldehyde dehydrogenase
Ethanol is a teratogen and alcohol consumption during pregnancy can result in the birth of a baby with fetal alcohol spectrum disorder (FASD) which may include physical, mental, behavioral and learning disabilities with lifelong implications.
Blood analysis: serum, plasma, or whole blood
• Higher concentrations in serum than in blood
•
Venipuncture site should be cleansed with an alcohol-free disinfectant (aqueous benzalkonium chloride)
•
Specimens should be capped to avoid evaporative loss.
Blood may be stored, when properly sealed, for 14 days at room temperature or at 4°C, with or without preservative.
•
Blood analysis: serum, plasma, or whole blood
•
For longer storage or for non-sterile postmortem specimens, sodium fluoride should be used as a preservative to
52
Chem PPT Flashcards, Unit 3
What ratio is breath alcohol analysis based on? Evidential breath alcohol measurements are based on the ratio of 2100:1 (blood/breath)
What is the deprivation period for breath alcohol analysis?
Before breath alcohol analysis, a deprivation period of at least 15 minutes is recommended to allow for clearance for any residual alcohol that may be present in the mouth from very recent drinking, use of alcohol-containing mouthwash, or vomiting of alcohol-rich gastric fluid.
What safeguard is there to ensure against mouth alcohol contamination? prevent a decrease or occasionally an increase (via fermentation) in ethanol concentration.
To measure ethanol in serum/plasma, the enzymatic assay is the method of choice
(alcohol dehydrogenase)
What is the use of urinalysis in ethanol analysis?
What are some analysis of Ethyl Glucuronide
(EtG) and Ethylsulfate (EtS)?
Where methanol is used?
Duplicate tests, performed 3 to 10 minutes apart, typically must agree within 20 mg/dL
(0.02%) as an additional safeguard against mouth alcohol contamination.
Detection of alcohol in urine represents ingestion of alcohol within the previous 8 to
12 hours.
Minor metabolites of ethanol
Ethyl glucuronide (EtG) may be detected as long as 80 hours after ethanol consumption and is found even when small amounts of alcohol are consumed.
Used as a marker of recent ethanol intake due to its long urinary elimination time and its specificity for ethanol exposure.
Commercial solvent for products such as deicers and windshield washer fluids. methanol Name the agent that cause cellular hypoxia and it is metabolized by liver alcohol dehydrogenase to formaldehyde?
Formaldehyde is subsequently oxidized to
…………….by aldehyde dehydrogenase.
Methanol causes what type of disorders?
Formic acid acidosis, optic neuropathy, resulting in blindness, or death if not treated promptly methanol Between ethanol and methanol which one oxidized slower?
What are the mainstay of therapy for methanol toxicity?
Administration of ethanol or fomepizole as a competitive alcohol dehyrogenase inhibitor
Administration of either folate/folinic acid
Dialysis
53
Chem PPT Flashcards, Unit 3
What percentage of aqueous isopropanol is rubbing alcohol?
What is isopropanol metabolized to? By which enzyme?
70% aqueous solution of isopropanol = rubbing alcohol
Isopropanol metabolized by alcohol dehydrogenase to acetone, which is eliminated much more slowly.
It has about twice the CNS depressant action as ethanol.
Does isopropanol have the same CNS depressant action as ethanol?
Does Acetone have CNS depressant activity? Yes
What is Ethylene Glycol commercially known as?
Antifreeze
Are the effects of Ethylene Glycol harmless?
What metabolite would be measured after ingesting Ethylene Glycol?
Relatively harmless initially, but metabolites like oxalic acid and glycolic acid can be lethal
Determination of ethylene glycol and glycolic acid provides useful clinical and confirmatory analytical information in cases of ethylene glycol ingestion
What is the treatment for accidental ingestion of
Ethylene Glycol?
Treatment: Administration of ethanol or fomepizole as a competitive alcohol dehydrogenase inhibitor dialysis and dialysis
What is Flame Ionization Gas Chromatography? It is the most common method used to detect and quantify volatile alcohols in biological samples. It distinguishes types of alcohol and measure concentrations as low as 10 mg/dL
(0.01%)
How are samples prepared for Flame Ionization
Gas Chromatography?
They are prepared by direct injection and headspace analysis.
Is a prescription required for acetaminophen? No
What type of drug is acetaminophen?
What can overdose of acetaminophen cause?
What is the treatment for acetaminophen overdose?
Antipyretic
Hepatic and renal toxicity
Administration of N-acetylcysteine (NAC)
What does Rumack-Matthew nomogram do? relates serum acetaminophen concentration and time after acute ingestion to the probability of hepatic necrosis
Spectrophotometry and immunoassays What are other screening methods for acetaminophen?
In the Rumack-Matthew Nomogram what hour increments are used on the x-axis?
What does the Rumack-Matthew line indicate in the Nomogram?
What is the treatment line in the Rumack-
Matthew Nomogram?
The x-axis are in 4 hour increments.
Any values (concentration) above the line may indicate hepatic damage.
The treatment line is 25% less than the rumack matthew line, which is the threshold for acetaminophen toxicity.
54
Chem PPT Flashcards, Unit 3
What is the common name for Acetylsalicylic acid?
What is Acetylsalicylic acid used for?
Aspirin
What does Acetylsalicylic acid enhance and inhibit?
How does one treat Acetylsalicylic acid toxicity?
What can be used to treat Acetylsalicylic acid?
Analgesic, antipyretic, antiinflammatory
Acetylsalicylic acid enhances anaerobic glycolysis but inhibit the Krebs cycle and transaminase enzymes.
Acetylsalicylic acid toxicity is treated by decreasing further absorption, increasing elimination, and correcting acid-base and electrolyte disturbances.
Activated charcoal (prevents absorption)
Alkaline diuresis (elimination)
NaHCO
3
(alleviates metabolic acidosis)
What is Trinder Tests?
What is Tricyclic Antidepressants?
What is the use of Antipsychotic Drugs?
What is the effect of Antipsychotic Drugs?
What methods are used to measure the concentration of Antipsychotic Drugs?
Spot Tests, HPLC, fluorescent polarization immunoassay, salicylate hydroxylasemediated photometry, gas and liquid chromatography methods.
• Represent a class of drugs frequently prescribed for the treatment of depression
•
Therapeutic mechanism is the blockade of neuronal reuptake of serotonin and/or norepinephrine
•
Measured by chromatographic methods or by immunoassay
Generally used for psychiatric and other disorders
Principal manifestation involve the CNS and cardiovascular system
Measured by chromatographic methods or by immunoassay
What is the use of Antihistamines? Used to treat allergies and aid sleep
Does Antihistamines available over the counter? Many available over the counter
What is Histamine role in the body? Histamine is released from mast cells and plays an important physiological role in
What is the function of Histamine? immediate hypersensitivity and allergic response
Histamine functions as a neurotransmitter in the CNS and stimulates gastric acid secretion.
What methods are used to determine Histamine concentration?
Spectrographic methods: GC-MS/MS, LC-
MS/MS
What are the characteristics of Antimuscarinic Synonymous with anticholinergic, inhibiting
55
Chem PPT Flashcards, Unit 3
Agents?
How does the Antimuscarinic agent go inside the body? the action of acetylcholine, a neurotransmitter in the parasympathetic nervous system.
What is the best use of Antimuscarinic drugs? Antimuscarinic drugs relax smooth muscle, decrease the secretion of saliva, sweat, and digestive juice, and dilate the pupil of the eye.
May be eaten or ingested in tea
Treatment: based on clinical presentation Is there any treatment involved for
Antimuscarinic?
What is Acetylcholine?
How do agents Related to the
Cholinergic Toxidrome act?
What is duration of acetylcholine action is controlled by?
Where is Acetylcholinesterase found?
Where is Butyrylcholinesterase found?
What is Organophosphate poisoning results from?
What is most commonly Organophosphate poisoning?
How does Organophosphates work?
What is the purpose of AChE? an essential neurotransmitter that affects parasympathetic synapses (autonomic and
CNS), sympathetic preganglionic synapses, and the neuromuscular junction by producing uncontrolled acetylcholine transmission through inactivation of cholinesterase enzymes or direct stimulation of acetylcholine receptors
-
Acetylcholinesterase
butyrylcholinesterase / pseudocholinesterase
in red blood cells
nervous tissue
skeletal muscle.
Butyrylcholinesterase is found in plasma, liver, heart, pancreas and brain. from exposure to organophosphates (OPs), which cause the inhibition of acetylcholinesterase (AChE), leading to the accumulation of acetylcholine (ACh) in the body results from exposure to insecticides or nerve agents
Organophosphates inhibit AChE, causing OP poisoning by phosphorylating the serine hydroxyl residue on AChE, which inactivates
AChE.
It’s critical for nerve function, so the irreversible blockage of this enzyme, that causes acetylcholine accumulation, results in muscle overstimulation.
What does diagnosis of organophosphate and carbamate toxicity depend on?
Mainly on exposure history, physical presentation, clinical suspicion, and laboratory support.
Administration of atropine, pralidoxime. What is the treatment for organophosphates and carbamate?
Measurement of organophosphates and carbamate?
Spectrophotometric measurement of acetylcholinesterase and butyrylcholinesterase
56
Chem PPT Flashcards, Unit 3
What are drugs of abuse?
What are the drugs of abuse?
What are the drugs mentioned above collectively known as? activity. GC-MS and GC-MS/MS of urine for organophosphate and carbamate metabolites?
Any drug that is repeatedly and deliberately used in a way other than prescribed or socially sanctioned.
1. Tricyclic antidepressants
2. Benzodiazepines
3. Barbiturates
4. Methadone
5. Methylenedioxymethamphetamine
(MDMA)
6. Methylenedioxyethylamphetamine
(MDEA)
7. Oxycodone
8. Amphetamines
9. Cocaine
10. Marijuana
11. Opiates
12. Phencyclidine
SAMHSA (Substance Abuse and Mental
Health Services Administration) or NIDA
(National Institute on Drug Abuse).
Department of Transportation. Under which department do the SAMHSA drug tests are required?
What are Barbiturates?
Barbiturates are also effective as?
What is one of the problems that you face when taking Barbiturates?
Barbiturates have now largely been replaced by benzodiazepines in routine medical practice – for example, in the treatment of anxiety and insomnia – mainly because?
They are drugs that act as central nervous system depressants, with wide spectrum of effects, from mild sedation to total anesthesia.
As analgesics, anxiolytics, hypnotics, and anticonvulsants.
They have addiction potential, both physical and psychological
Because benzodiazepines are significantly less dangerous in overdose as there is no specific antidote for barbiturate overdose.
In what cases are Barbiturates still being used? Barbiturates are still used in general anesthesia, for epilepsy, for the treatment of acute migraines and cluster headaches.
What are some of the barbiturates confirmatory test ?
What are the immunoassay techniques ?
Gc with flame Ionization detection,
Nitrogen phosphorus detection, mass spectrometry MS detection capillary electro
Screening test
What is the main action of barbiturates ?
What are some main uses of barbiturates?
Suppression of cns sedative-hypnotic drugs
Induce anesthesia, treat seizures, decreased
57
Chem PPT Flashcards, Unit 3
Are barbiturates acids or bases? Weak or strong?
What are the active duration time of long acting
?
Which class of of drugs sometimes called benzos are a class of psychoactive drugs that enhanced effect of the neutransmitter gammaaminobutyric?
If used alone, do benzodiazepines commonly cause fatal cns depression ?
True or false the prototype benzodiazepines are diazepam nordiazepam (n-desmethyldiazepam)
Uses of benzodiazepine.
Classification of Benzodiazepines.
Confirmatory tests of Benzodiazepines
Where is the Cannabinoids derived from?
What are the effects of Cannabinoids?
Which is the primary psychoactive component of Cannabinoids?
Cannabinoids measure by which specimen? intracranial pressure, enthanasia
Weak acids
2-6 days
48-52
Benzodiazepines
No but at high doses will cause hyponosis and stupor
True useful in treating anxiety, insomnia, agitation, seizures, muscle spasms, alcohol withdrawal and as a premedication for medical or dental procedures.
They are categorized as either short-, intermediate-, or long-acting. Short- and intermediate-acting benzodiazepines are preferred for the treatment of insomnia; longer-acting benzodiazepines are recommended for the treatment of anxiety.
-extraction procedures by Liquid-Liquid extraction or Solid-Phase extraction, analysis of urine specimens by GC-MS, LC with UV detection, LC-MS and LC-MS/MS.
Cnnabinoids found in the marijuana plant
C annabis sativa
Psychotropic effects are euphoria, distorted perception, relaxation and a feeling of wellbeing.
Delta-9-tetrahydrocannabinol (THC) is the primary psychoactive component
Delta-9-tetrahydrocannabinol (THC), which is measured in urine. which methods used to measure Cannabinoids? presumptive positive result using immunoassay method should be confirmed by
Where is the Opiates derived from? quantitative GC-MS/MS or LC-MS/MS
Opiates found naturally in the opium poppy plant Papaver somniferum .
Which Form of Opiates is better In medical?
What are primarily used in medicine for the
In a medical context the term usually indicates medications that are artificially made rather than extracted from opium.
Opioids
58
Chem PPT Flashcards, Unit 3 treatment of pain? sedation, respiratory depression, constipation, and a strong sense of euphoria are side effects of what?
Opioid ? can develop with ongoing administration, leading to a withdrawal syndrome with abrupt discontinuation. opioids dependence
Opios can cause death in overdose from what? respiratory depression.
Opioids work by binding to opioid receptors, which are found principally iwhere? These the central and peripheral nervous system and the gastrointestinal tract. receptors mediate both the psychoactive and the somatic effects of opioids
Although the term opiate is often used as a
Papaver somniferum (opium poppy), synonym for opioid, the term opiate is properly limited to the natural alkaloids found in the resin of the___?___ while opioid refers to synthetic substances
Natural Opium Alkaloidsare Phenanthrenes which include what?
Natural Opium Benzylisoquinolines are what?
Poppy seeds (Papaver somniferum) are a type of natural _____?___
Semisynthetic Opiates are what?
Morphine
Codeine
Thebaine
Papaverine
Noscapine
Opium
Name the 5 Fully Synthetic Opioids.
Name the 3 antagonist to Opioids.
What method of choice is used for urine screening?
Heroin
Hydrocodone
Hydromorphone
Oxycodone
Oxymorphone
Fentanyl
Meperidine
Methadone
Propoxyphene
Tramadol
Buprenorphine
Naloxone
Naltrexone
Immunoassay
What are the 3 Confirmatory test used for
Opioids
Sympathomimetic drugs are stimulant
Gas chromatography mass spectrometry
(GC-MS)
Liquid chromatography mass spectrometry
(LC-MS)
Liquid chromatography Tandem Mass
Spectrometry (LC-MS/MS)
Effects of agonists of the sympathetic nervous
59
Chem PPT Flashcards, Unit 3 compounds which mimic the what effects?
Give some examples of catecholamines.
Sympathomimetic drugs are used to treat what?
List some drugs considered to have a high potential of abuse
What is Amphetamine and Methamphetamine primary action? system such as the catecholamines. epinephrine (adrenaline) norepinephrine (noradrenaline) dopamine
Cardiac arrest, low blood pressure, or even delay premature labor, among other things.
Amphetamine, Methamphetamine,
Ephedrine, Pseudoephedrine,
Phenylpropanolamine
To increase dopamine, serotonin, norepinephrine (extracellular monoamine neurotransmitters); Also increase blood pressure, heart rate, body temperature and motor activity, relaxed bronchial muscle and depress the appetite.
Central and peripheral nervous system.
In plants of Ephedra genus
What is the main system Amphetamine and
Methamphetamine affect?
Where do Ephedrine and Pseudoephedrine naturally occurs?
What kind of receptor is Ephedrine?
Explain the mechanism of Ephedrine
How is Pseudoephedrine primarily used?
Ephedrine is both an α- and β- adrenergic receptor agonist
It enhances the release of norepinephrine from sympathetic neurons and is considered a mixed-acting sympathomimetic drug causing hypertension, tremors, myocardial infarction, seizures and stroke.
As a decongestant because of its vasoconstrictive properties (alpha adrenergic action), and as a precursor for the illicit synthesis of methamphetamine.
Name a psychoactive drug of the phenethylamine and amphetamine chemical classes which is used as a stimulant, decongestant, and anorectic agent.
Phenylpropanolamine (PPA)
Phenylpropanolamine (PPA) is a metabolite of? Ephedrine and pseudoephedrine
PPA is commonly used in prescription and cough and cold preparations. over-the-counter for?
Designer Amphetamines are also known as?
“club drugs” or “designer drugs”
Short-term effects of designer amphetamines include:
Long-term effects of designer amphetamines include: euphoria, energy, desire for social interaction, distortion of visual and auditory sensation
Serotonin syndrome
Hepatotoxicity
Neurotoxicity
Psychopathology
60
Chem PPT Flashcards, Unit 3
What psychostimulant used to treat attention deficit hyperactivity disorder and narcolepsy?
What drugs has clinical effects similar to those of amphetamines
Because of stimulant and purported aphrodisiac properties, increasingly subject to diversion and abuse, what drugs is this?
What do you call a drug that is chemically alkaloid methylbenzoylecgonine found in
Erythroxylum coca
A kind of drug used for local anesthesia and vasoconstriction in nasal surgery, and to dilate pupils in ophthalmology?
What is this form of cocaine that is administered by nasal insufflation or snorting?
What is this form of cocaine that is heated and its vapors inhaled?
What drug is a potent CNS stimulant that elicits a state of increased alertness and euphoria
(same as amphetamine but of shorter duration)?
What analyte of choice in screening for cocaine use?
What is the initial screening method by choice for cocaine use?
LSD is structurally similar to________.
A.
Melatonin
B.
ADH
C.
Serotonin
D.
Epinepherin
LSD causes several psychological effects.
These include:
A.
B.
perceptual distortions of color depersonalization
C. loss of body image
D. All of the above
LSD is made from bacteria. True or False.
A.
True
B.
False
Current ways of use for LSD include:
A.
Postage Stamps
B.
Gel Caps
C.
Tablets
D.
All the above
Current detection methods for LSD as an analyte include:
Abuse
Methylphenidate (Ritalin)
Methylphenidate (Ritalin)
Methylphenidate (Ritalin)
Cocaine
Cocaine hydrochloride salt (powder) freebase known as crack cocaine
Cocaine
Benzoylecgonine (BE)
Initial screening is by immunoassay detection
GC-MS is the confirmatory method of choice
C
D
B
D
D
61
Chem PPT Flashcards, Unit 3
A.
GC-MS/MS
B.
LC-MS/MS
C.
LC-MS
D.
All of the Above
Can drugs facilitate sexual assault?
Yes or No
A.
Yes
B.
No
Current drugs that DFSA(defined as the use of alcohol, drugs, and/or chemical agents to incapacitate an individual can facilitate sexual assault) include:
A. Alcohol
B. Benzodiazepine
C.Chloral hydrate
D. All of the above
All or most athletes are required to undergo drug screening. The procedure is defined as:
A.
Drug screening
B.
In competition testing
C.
Prescreening
D.
Out of competition testing
Drug abuse can be detected using several samples taken from patients.
Choose the answer you think is correct.
A.
Blood, Plasma, Serum
B.
Meconium, Hair, Sweat
C.
Oral Secretions(Saliva)
D.
All of the above
Heavy metal poisoning is ___
Symptoms and physical findings associated with heavy metal poisoning
Name 5 heavy metals, essential to body function in very small amounts. But, if these metals accumulate in the body in concentrations sufficient to cause poisoning, then serious damage may occur
The heavy metals most commonly associated with poisoning of humans are
Heavy metal poisoning may occur as a result of
(6 ways).
What is AA-F?
What is AA-ETA?
A
D
D
D the accumulation of heavy metals, in toxic amounts, in the soft tissues of the body vary according to the metal accumulated zinc, copper, chromium, iron and manganese lead, mercury, arsenic and cadmium industrial exposure, air or water pollution, foods, medicines, improperly coated food containers, or the ingestion of lead-based paints
Atomic absorption spectrometry with flame
Atomic absorption spectrometry with
62
Chem PPT Flashcards, Unit 3
What is ICP-ES?
What is ICP-MS?
What is LC-ICP/MS? electrothermal atomization furnace
Inductively coupled plasma emission spectrometry
Inductively coupled plasma mass spectrometry
High performance liquid chromatography inductively coupled mass spectrometry
Aluminum toxicity Patients with renal failure are candidates for what?
Aluminum avidly binds to proteins such as?
Aluminum has been implicated in which disease?
What accumulates in blood if not filtered by kidney?
Aluminum is neurotoxic, targeting the central nervous system, which can lead to?
The primary side-effect or "wake-up call" that indicates aluminum has intoxicated the brain is a very serious condition called?
Symptoms of aluminum hypersensitivity include:
Transferrin
Alzheimer's Disease
Aluminum
Serious immunological and neurodegenerative disorders
HYPERSENSITIVITY
-Heightened sensitivity to light or darkness.
-Abnormal sensitivity to hot and cold temperatures.
-An aversion to noise, touch, movement, odors, etc.
-Unexplained feelings of apprehension or uneasiness.
-Feelings of inferiority, embarrassment or shame.
-Feelings of irritability, agitation or annoyance.
-Those who are easily frightened or alarmed sometimes become overly disturbed and provoked, displaying irrational outbursts of anger, road rage, and bad temper
Aluminum hypersensitivity What metal can cause the following symptoms, becoming easily frightened or alarmed sometimes become overly disturbed and provoked, displaying irrational outbursts of anger, road rage, and bad temper
What are the sensory symptoms caused by hypersensitivity to aluminum?
What are the emotional symptoms caused by hypersensitivity to aluminum?
Overly sensitive to all of the five senses.
-Heightened sensitivity to light or darkness.
-Sensitivity to hot and cold.
-Aversion to noise, touch, odors, movements.
Emotional variance of all types of negative emotions.
-Unexplained feelings of apprehension, dread,
63
Chem PPT Flashcards, Unit 3
What are some industries were exposure to aluminum can happen? uneasiness
-feelings of shame, embarrassment, and inferiority
-irritability annoyance and and agitation
-these feelings can become violent.
1) Mining industry
2) Factory work
3) Welding agriculture
What is another source of aluminum exposure? Aluminum vapors are ingested every time the nose catches cigarette smoke wafting by.
What foods can lead to aluminum exposure? 1) Baking powder
2) Self-rising flour
3) Salt
4) Baby formula
5) Coffee creamers
6) Baked and processed foods
Coloring and caking agents
What drugs are sources of aluminum exposure?
What vaccines are sources of aluminum exposure?
What cosmetics and personal care products are sources of aluminum exposure?
What are some other sources of aluminum exposure?
1) Antacids
2) Analgesic
3) Anti-diarrheals
Additives such as magnesium stearate
1) Hepatitis A and B
2) Hib
3) DTap
4) Pneumococcal vaccine
Gardasil (HPV) and others
1) Antiperspirants
2) Deodorants
3) Lotions
4) Sunscreens shampoos
Aluminum products including
1) foil
2) cans
3) juice pouches
4) tins
5) water bottles
378 Red meats cooked in aluminum foil showed an increase in aluminum by 89 to ___ percent.
Poultry is increased by 76 to ____ percent when cooked in aluminum foil.
Aluminum levels increase with ___ (longer/higher) cooking temperatures and longer cooking times.
How can Antimony toxicity occur?
214 higher
Through occupational exposure or during
64
Chem PPT Flashcards, Unit 3
What can occupational exposure cause?
True or False?
Antimony trioxide is possibly carcinogenic to humans
What can antimony be used for therapeutically? Treatment of leishmaniasis and schistosomiasis
What is the major toxic side-effect of antimonials as a result of therapy?
Cardiotoxicity and pancreatitis
HIV and visceral leishmaniasis co-infections Where are the above side-effects mostly seen in?
How/where can antimony be measured in? Urine, feces, and blood
It is highly toxic in its inorganic form. Arsenic is a heavy metal. It exists in compounds that may be organic or inorganic. Which form is toxic?
Why is arsenic toxic? therapy
Respiratory irritation, pneumoconiosis, antimony spots on skin, and gastrointestinal symptoms
True
What is an effective antidote? on many cell enzymes, which affect metabolism and DNA repair
British anti-lewisite (BAL), The active agent in BAL is dimercaprol, a sulfhydryl-reducing agent.
It is Arsenic (As) What is the one of the most widely known toxic metal?
What are the most common routes of exposure to arsenic?
Where are major sources of inhaled arsenic may come from?
Ingestion and inhalation are the most common routes of exposure to arsenic, and they are the routes that most commonly lead to illness
Major sources of inhaled arsenic may come from air emissions from burning of fossil fuels that contain arsenic, cotton gins, glass manufacturing operations, pesticide manufacturing facilities, smelters, and tobacco smoke
Where is arsenic found?
What is shown to have high levels of inorganic
Arsenic?
What is the effect of Beryllium poisoning?
What is the toxicity of Beryllium?
Meat, fish, and poultry account for 80% of dietary arsenic intake. Fish, bivalve shellfish, and algae also contain arsenic in the form of arsenobetaine and arsenocholine, sometimes referred to as "fish arsenic."
Recent studies have shown one form of seaweed, hijiki, to contain high levels of inorganic arsenic
Beryllium poisoning is illness resulting from the toxic effect of beryllium in its elemental form or in various chemical compounds
The toxicity of beryllium depends upon the duration, intensity and frequency of exposure
65
Chem PPT Flashcards, Unit 3
What are some uses of Beryllium in everyday life?
What is Chronic berylliosis?
(features of dose), as well as the form of beryllium and the route of exposure (i.e. inhalation, dermal, ingestion).
It has been used in electronics, ceramics, research and development labs, aircraft, and the atomic energy and defense industry
It is a pulmonary and systemic granulomatous disease
What is the cause of Chronic berylliosis? Exposure to beryllium.
What is the form of Acute beryllium disease? Chemical pneumonitis.
Is the quantification of Beryllium in serum or urine useful in making the diagnosis?
No, air analysis (TLV threshold limit value), is the preferred method of exposure evaluation.
Cadmium (Cd) Nam specific metal which is Byproduct of zinc and lead smelting?
What does breathing the fumes of Cd vapors lead to?
Nasal epithelial deterioration and pulmonary congestion resembling chronic emphysema.
What is a common source of chronic exposure? Spray painting of organic base paints without the use of protective breathing apparatus
Auto repair mechanics represent a workgroup that has significant opportunity for exposure to -
Cadmium (Cd)
What does exposure to Cadmium cause?
What is a side effect of tobacco smoke?
At what concentration would you observe acute toxicity by Cadmium?
By what methods would you be able to quantify
Cadmium?
Cd toxicity present with renal dysfunction with proteinuria. Chronic exposure causes accumulated renal damage.
Moderately increased blood Cadmium
If blood concentration exceeds 50 ng/mL
Cadmium is usually quantified by atomic absorption spectrometry, but it also has been accurately quantified by ICP-MS
Chromium Occupational exposure to which element is considered hazardous?
Which work industries use Chromium?
What is the toxic form of Cr?
Which would be the best method of analyzing
Chromium?
What is an essential cofactor for vitamin B
12
?
What are symptoms of acute Cobalt exposure?
What kind of sample is usually used to
Used in the manufacture of stainless steel, chrome plating, tanning of leather, as a dye for printing and textile manufacture, as a cleaning solution, as an anticorrosive agent in cooling systems, and in metallic orthopedic implants.
Toxic form of Cr is Cr6+.
ICP MS is the preferred technology for quantification of chromium in body fluids
Cobalt.
Cardiomyopathy and Renal Failure.
Urine.
66
Chem PPT Flashcards, Unit 3 determine excessive exposure of cobalt?
Where is cobalt usually found?
How is cobalt quantified in biological tissues? It is quantified by atomic absorption
What is Copper (Cu)? spectrometry or by ICP-MS.
•
Found in common pesticides, marine antifouling paints, and wood preservatives
• Ingestion of copper produces gastrointestinal symptoms, hemolytic anemia, hepatitis with jaundice, and renal damage.
Deficiency in Copper cause what Disease?
They are found in metal alloys that are hard and resistant to corrosion.
What is the specimen of choice for diagnosis
Wilson Disease?
Where does Copper circulate in the body?
What is Gadolinium (Gd)?
The classical presentation of Cu toxicosis is represented by the genetic disease of Cu accumulation known as Wilson's disease. This disease is typified by hepatocellular damage
(increased transferases) and/or changes in mood and behavior caused by accumulation of
Cu in central neurons.
Evaluation of serum and urine copper concentration is useful in diagnosing Wilson's disease.
Since copper circulating in blood is bound to ceruloplasmin and ceruloplasmin formation is decreased in Wilson's disease, serum copper concentration is less than the reference interval for serum, and urinary copper concentrations are increased.
A chemical element found in image contrast agents used during Magnetic Resonance
Imaging (MRI) and Magnetic Resonance
Angiography (MRA) procedures.
What is the name of an agent involved in nephrogenic systemic fibrosis?
When will be the most consistent risk factor in
GBCA?
How is GBCA excreted?
How Lead (Pb) has been found in the environment?
Where is Lead (Pb) commonly found?
Gadolinium based contrast agent (GBCA) is thought to be involved in nephrogenic systemic fibrosis.
Exposure to GBCA during a condition of low glomerular filtration rate appears to be the most consistent risk factor.
Because GBCA is excreted by the kidney, exposure is prolonged in patients with renal insufficiency.
Commonly found in the environment that is an acute and chronic toxin.
Common in paints, ceramics, leaded gasoline in automobiles and in soil.
67
Chem PPT Flashcards, Unit 3
How does a person exposure to Lead?
What is the main reason of measuring blood
Pb?
What is the Pb concentration in adults to be considered of significant exposure?
Exposure to Pb is through ingestion, inhalation or dermal --contact.
The definitive test for lead toxicity is measurement of blood Pb
WHO has defined blood Pb concentrations
>30 µg/dL in adults as indicative of significant exposure
Is >60 µg/dL
What is the Lead concentrations required chelation therapy
What is analysis of Lead routinely performed by?
What is the specimen of choice for lead analysis?
What are other methods can be used in urine quantification?
What is Manganese?
ICP MS, electrothermal atomic absorption spectrometry, or anodic stripping voltammetry
EDTA blood
Urinalysis
How can you get toxicity from Manganese?
Ubiquitous in environment used as a binding agent in red bricks, An anti-corrosive in most steel alloys, a cleaning agent for glassware, and a common pigment in paints and glazes.
Toxicity comes as a result of exposure from dust from mining, ore crushing, machining of alloys, manufacture or destruction of bricks.
What can accumulation of Manganese in the brain cause?
What can be tested for indication of Manganese in the body?
What is Mercury commonly known as?
What was Mercury formerly named as?
What is Mercury?
Manganism (Parkinson-like neurodegenerative disorder).
Blood and urine concentrations are good indicators of exposure.
Quicksilver
Hydrargyrum
A heavy, silvery d-block element, mercury is the only metallic element that is liquid at standard conditions for temperature and pressure.
What is Mercury used in? Mercury is used in thermometers, barometers, manometers, sphygmomanometers, float valves, mercury switches, mercury relays, fluorescent lamps.
What is Mercury replaced by? Replaced by alcohol- or galinstan-filled glass thermometers and thermistor- or infraredbased electronic instruments.
What are some applications for use of mercury? Clinical scientific research application data
Dental restoration What is mercury used for as an almalgam material?
What is the name of a medical condition caused by exposure to mercury?
Toxic effects of Mercury
Hydrargyria
Includes damage to the brain, kidneys and lungs. Mercury poisoning can result in several
68
Chem PPT Flashcards, Unit 3
Symptoms of Mercury.
What is the used of Nickel (Ni)?
What happened when Nickle exposed to air?
Uses Platinum (Pt) in chemotherapy. diseases, including acrodynia (pink disease),
Hunter-Russell syndrome, and Minamata disease.
It includes sensory impairment (vision, hearing, speech), disturbed sensation and a lack of coordination. The type and degree of symptoms exhibited depend upon the individual toxin, the dose, and the method and duration of exposure.
Used in the production of metal alloys, Nibased rechargeable batteries, and as a catalyst in the hydrogenation of oils
Patients exposed to Ni carbonyl exhibit rapid onset of pulmonary congestion and inability to oxygenate hemoglobin, followed by development of lesions of the lung, liver, kidney, adrenal glands and spleen.
Pt-containing anti-neoplastic agents, cisplatin and carboplatin are used in chemotherapy
-Cause nephrotoxicity
-Can induce neutropenia
-Can induce renal failure if administered with nephrotoxic antibiotics
Measured by AA-ETA and ICP-MS oxidative damage.
Platinum (Pt) measured by Which methods?
Selenium is a Essential element that may play a role in mitigating biological damage caused by what?
Selenium is a cofactor required to maintain what type of activity?
What type of muscle is the most susceptible to selenium deficiency ?
Name one type of Symptom caused by Se poisoning
Selenium deficiency is also related to what?
What is the most abundant element in earth's environment (26% of earth's crust)
Silicon toxic forms include what? glutathione peroxidase
Cardiac (cardiomyopathy) hair loss, muscle cramps, nausea, vomiting, diarrhea, joint pain, fatigue, fingernail changes and blistering skin total parenteral nutrition silicon asbestos and silicone asbestos bodies Inhalation of asbestos containing dust leads to the position of asbestos fibers in the pulmonary alveoli causing asbestosis with the presence of what?
Diagnosis of silicon toxicity can be made by chest radiograph and presence in sputum and
69
Chem PPT Flashcards, Unit 3 what type of tests?
How does dangerous exposure to asbestos occur?
What is asbestosis?
What does asbestosis cause?
What are pleural plaques?
What significantly increases lung cancer?
What is the Silver analysis limited to?
What is argyria?
When is argyria produced? lung biopsy
When materials containing the fibers are disturbed.
Fibers accumulate in the lungs’ narrow branches, inflamming, and scarring airways.
Chronic cough and chest pain
The needle-shapes fibers may also migrate into the pleural lining. Pleura becomes inflamed, plaques builds up and may restrict breathing.
Smoking
Silver analysis is limited to monitoring of burn patients treated with silver sulfadiazine, and monitoring of patients treated with silvercontaining nasal decongestants. graying of the skin
Argyria is produced when Silver (Ag) is deposited in many organs including the sub- epithelium of the skin and mucous membranes
Thallium (Tl) Which toxic metal is a byproduct of lead smelting, coal combustion, and cement manufacture?
Which toxic metal is formerly used in rodent poisons?
Thallium is absorbed by____(1)____, ,
___(2)____and ___(3)____
What are the effects of exposure to high doses of thallium?
What toxic metal is the 9th most abundant element in the earth's crust?
Thallium (Tl)
1) ingestion
2) inhalation
3) skin contact hair loss, peripheral neuropathy and seizures, and renal failure
Titanium
If ingested, titanium is rapidly excreted where? urine and stool
True or False False(nonirritating and almost completely
Titanium dust is irritating and almost completely fibrogenic. nonfibrogenic)
Where does titanium-containing alloys being used?
What allows osseointegration? in artificial joints, prosthetic devices, and implants
Titanium dioxide
True True or False
Concerning titanium, serum concentrations are used to monitor prosthesis degradation.
Vanadium is not a recognized occupational hazard. True or False
A.
True
B.
False
Vanadium is a byproduct of refining,
A
D
70
Chem PPT Flashcards, Unit 3
Specifically:
A.
Iron
B.
Titanium
C.
Uranium
D.
All of the Above
A clinically significant reaction of
Vanadium by the human body includes:
A.
Green tongue
B.
Runny Nose
C.
Green blood at venipuncture
D.
Discoloration of Nails
A patient sample has a flag for Vanadium, a clinically significant toxin. What are the possible causes for the elevated serum concentrations present?
A.
Compromised renal function and dialysis
B.
Joint replacement
C.
Prosthesis degradation
D.
All of the above
A
D
71