Physics - Grades Nine Through Twelve
advertisement

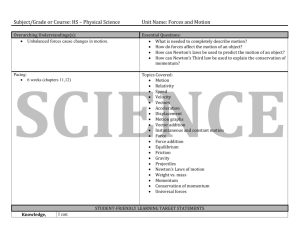
Physics - Grades Nine Through Twelve Science Content Standards. Standards that all students are expected to achieve in the course of their studies are unmarked. Standards that all students should have the opportunity to learn are marked with an asterisk (*). Motion and Forces 1. Newton's laws predict the motion of most objects. As a basis for understanding this concept: a. Students know how to solve problems that involve constant speed and average speed. b. Students know that when forces are balanced, no acceleration occurs; thus an object continues to move at a constant speed or stays at rest (Newton's first law). c. Students know how to apply the law F=ma to solve one-dimensional motion problems that involve constant forces (Newton's second law). d. Students know that when one object exerts a force on a second object, the second object always exerts a force of equal magnitude and in the opposite direction (Newton's third law). e. Students know the relationship between the universal law of gravitation and the effect of gravity on an object at the surface of Earth. f. Students know applying a force to an object perpendicular to the direction of its motion causes the object to change direction but not speed (e.g., Earth's gravitational force causes a satellite in a circular orbit to change direction but not speed). g. Students know circular motion requires the application of a constant force directed toward the center of the circle. h. * Students know Newton's laws are not exact but provide very good approximations unless an object is moving close to the speed of light or is small enough that quantum effects are important. i. * Students know how to solve two-dimensional trajectory problems. j. * Students know how to resolve two-dimensional vectors into their components and calculate the magnitude and direction of a vector from its components. k. * Students know how to solve two-dimensional problems involving balanced forces (statics). l. * Students know how to solve problems in circular motion by using the formula for centripetal acceleration in the following form: a=v2/r. 1 m. * Students know how to solve problems involving the forces between two electric charges at a distance (Coulomb's law) or the forces between two masses at a distance (universal gravitation). Conservation of Energy and Momentum 2. The laws of conservation of energy and momentum provide a way to predict and describe the movement of objects. As a basis for understanding this concept: a. Students know how to calculate kinetic energy by using the formula E=(1/2)mv2 . b. Students know how to calculate changes in gravitational potential energy near Earth by using the formula (change in potential energy) =mgh (h is the change in the elevation). c. Students know how to solve problems involving conservation of energy in simple systems, such as falling objects. d. Students know how to calculate momentum as the product mv. e. Students know momentum is a separately conserved quantity different from energy. f. Students know an unbalanced force on an object produces a change in its momentum. g. Students know how to solve problems involving elastic and inelastic collisions in one dimension by using the principles of conservation of momentum and energy. h. * Students know how to solve problems involving conservation of energy in simple systems with various sources of potential energy, such as capacitors and springs. Heat and Thermodynamics 3. Energy cannot be created or destroyed, although in many processes energy is transferred to the environment as heat. As a basis for understanding this concept: a. Students know heat flow and work are two forms of energy transfer between systems. b. Students know that the work done by a heat engine that is working in a cycle is the difference between the heat flow into the engine at high temperature and the heat flow out at a lower temperature (first law of thermodynamics) and that this is an example of the law of conservation of energy. c. Students know the internal energy of an object includes the energy of random motion of the object's atoms and molecules, often referred to as thermal energy. The greater the temperature of the object, the greater the energy of motion of the atoms and molecules that make up the object. d. Students know that most processes tend to decrease the order of a system over time and that energy levels are eventually distributed uniformly. e. Students know that entropy is a quantity that measures the order or disorder of a system and that this quantity is larger for a more disordered system. 2 f. * Students know the statement "Entropy tends to increase" is a law of statistical probability that governs all closed systems (second law of thermodynamics). g. * Students know how to solve problems involving heat flow, work, and efficiency in a heat engine and know that all real engines lose some heat to their surroundings. Waves 4. Waves have characteristic properties that do not depend on the type of wave. As a basis for understanding this concept: a. Students know waves carry energy from one place to another. b. Students know how to identify transverse and longitudinal waves in mechanical media, such as springs and ropes, and on the earth (seismic waves). c. Students know how to solve problems involving wavelength, frequency, and wave speed. d. Students know sound is a longitudinal wave whose speed depends on the properties of the medium in which it propagates. e. Students know radio waves, light, and X-rays are different wavelength bands in the spectrum of electromagnetic waves whose speed in a vacuum is approximately 3×108 m/s (186,000 miles/second). f. Students know how to identify the characteristic properties of waves: interference (beats), diffraction, refraction, Doppler effect, and polarization. Electric and Magnetic Phenomena 5. Electric and magnetic phenomena are related and have many practical applications. As a basis for understanding this concept: a. Students know how to predict the voltage or current in simple direct current (DC) electric circuits constructed from batteries, wires, resistors, and capacitors. b. Students know how to solve problems involving Ohm's law. c. Students know any resistive element in a DC circuit dissipates energy, which heats the resistor. Students can calculate the power (rate of energy dissipation) in any resistive circuit element by using the formula Power = IR (potential difference) × I (current) = I2R. d. Students know the properties of transistors and the role of transistors in electric circuits. e. Students know charged particles are sources of electric fields and are subject to the forces of the electric fields from other charges. f. Students know magnetic materials and electric currents (moving electric charges) are sources of magnetic fields and are subject to forces arising from the magnetic fields of other sources. 3 g. Students know how to determine the direction of a magnetic field produced by a current flowing in a straight wire or in a coil. h. Students know changing magnetic fields produce electric fields, thereby inducing currents in nearby conductors. i. Students know plasmas; the fourth state of matter, contain ions or free electrons or both and conduct electricity. j. * Students know electric and magnetic fields contain energy and act as vector force fields. k. * Students know the force on a charged particle in an electric field is qE, where E is the electric field at the position of the particle and q is the charge of the particle. l. * Students know how to calculate the electric field resulting from a point charge. m. * Students know static electric fields have as their source some arrangement of electric charges. n. * Students know the magnitude of the force on a moving particle (with charge q) in a magnetic field is qvB sin(a), where a is the angle between v and B (v and B are the magnitudes of vectors v and B, respectively), and students use the righthand rule to find the direction of this force. o. * Students know how to apply the concepts of electrical and gravitational potential energy to solve problems involving conservation of energy. 4 HS-PS1 Matter and Its Interactions HS-PS1 Matter and Its Interactions Students who demonstrate understanding can: HS-PS1-1. Use the periodic table as a model to predict the relative properties of elements based on the patterns of electrons in the outermost energy level of atoms. [Clarification Statement: Examples of properties that could be predicted from patterns could include reactivity of metals, types of bonds formed, numbers of bonds formed, and reactions with oxygen.] [Assessment Boundary: Assessment is limited to main group elements. Assessment does not include quantitative understanding of ionization energy beyond relative trends.] HS-PS1-2. Construct and revise an explanation for the outcome of a simple chemical reaction based on the outermost electron states of atoms, trends in the periodic table, and knowledge of the patterns of chemical properties. [Clarification Statement: Examples of chemical reactions could include the reaction of sodium and chlorine, of carbon and oxygen, or of carbon and hydrogen.] [Assessment Boundary: Assessment is limited to chemical reactions involving main group elements and combustion reactions.] HS-PS1-3. Plan and conduct an investigation to gather evidence to compare the structure of substances at the bulk scale to infer the strength of electrical forces between particles. [Clarification Statement: Emphasis is on understanding the strengths of forces between particles, not on naming specific intermolecular forces (such as dipole-dipole). Examples of particles could include ions, atoms, molecules, and networked materials (such as graphite). Examples of bulk properties of substances could include the melting point and boiling point, vapor pressure, and surface tension.] [Assessment Boundary: Assessment does not include Raoult’s law calculations of vapor pressure.] HS-PS1-4. Develop a model to illustrate that the release or absorption of energy from a chemical reaction system depends upon the changes in total bond energy. [Clarification Statement: Emphasis is on the idea that a chemical reaction is a system that affects the energy change. Examples of models could include molecular-level drawings and diagrams of reactions, graphs showing the relative energies of reactants and products, and representations showing energy is conserved.] [Assessment Boundary: Assessment does not include calculating the total bond energy changes during a chemical reaction from the bond energies of reactants and products.] HS-PS1-5. Apply scientific principles and evidence to provide an explanation about the effects of changing the temperature or concentration of the reacting particles on the rate at which a reaction occurs. [Clarification Statement: Emphasis is on student reasoning that focuses on the number and energy of collisions between molecules.] [Assessment Boundary: Assessment is limited to simple reactions in which there are only two reactants; evidence from temperature, concentration, and rate data; and qualitative relationships between rate and temperature.] HS-PS1-6. Refine the design of a chemical system by specifying a change in conditions that would produce increased amounts of products at equilibrium.* [Clarification Statement: Emphasis is on the application of Le Chatlier’s Principle and on refining designs of chemical reaction systems, including descriptions of the connection between changes made at the macroscopic level and what happens at the molecular level. Examples of designs could include different ways to increase product formation including adding reactants or removing products.] [Assessment Boundary: Assessment is limited to specifying the change in only one variable at a time. Assessment does not include calculating equilibrium constants and concentrations.] HS-PS1-7. Use mathematical representations to support the claim that atoms, and therefore mass, are conserved during a chemical reaction. [Clarification Statement: Emphasis is on using mathematical ideas to communicate the proportional relationships between masses of atoms in the reactants and the products, and the translation of these relationships to the macroscopic scale using the mole as the conversion from the atomic to the macroscopic scale. Emphasis is on assessing students’ use of mathematical thinking and not on memorization and rote application of problem-solving techniques.] [Assessment Boundary: Assessment does not include complex chemical reactions.] HS-PS1-8. Develop models to illustrate the changes in the composition of the nucleus of the atom and the energy released during the processes of fission, fusion, and radioactive decay. [Clarification Statement: Emphasis is on simple qualitative models, such as pictures or diagrams, and on the scale of energy released in nuclear processes relative to other kinds of transformations.] [Assessment Boundary: Assessment does not include quantitative calculation of energy released. Assessment is limited to alpha, beta, and gamma radioactive decays.] 5 HS-PS2 HS-PS2 Motion and Stability: Forces and Interactions Motion and Stability: Forces and Interactions Students who demonstrate understanding can: HS-PS2-1. Analyze data to support the claim that Newton’s second law of motion describes the mathematical relationship among the net force on a macroscopic object, its mass, and its acceleration. [Clarification Statement: Examples of data could include tables or graphs of position or velocity as a function of time for objects subject to a net unbalanced force, such as a falling object, an object rolling down a ramp, or a moving object being pulled by a constant force.] [Assessment Boundary: Assessment is limited to one-dimensional motion and to macroscopic objects moving at non-relativistic speeds.] HS-PS2-2. Use mathematical representations to support the claim that the total momentum of a system of objects is conserved when there is no net force on the system. [Clarification Statement: Emphasis is on the quantitative conservation of momentum in interactions and the qualitative meaning of this principle.] [Assessment Boundary: Assessment is limited to systems of two macroscopic bodies moving in one dimension.] HS-PS2-3. Apply scientific and engineering ideas to design, evaluate, and refine a device that minimizes the force on a macroscopic object during a collision.* [Clarification Statement: Examples of evaluation and refinement could include determining the success of the device at protecting an object from damage and modifying the design to improve it. Examples of a device could include a football helmet or a parachute.] [Assessment Boundary: Assessment is limited to qualitative evaluations and/or algebraic manipulations.] HS-PS2-4. Use mathematical representations of Newton’s Law of Gravitation and Coulomb’s Law to describe and predict the gravitational and electrostatic forces between objects. [Clarification Statement: Emphasis is on both quantitative and conceptual descriptions of gravitational and electric fields.] [Assessment Boundary: Assessment is limited to systems with two objects.] HS-PS2-5. Plan and conduct an investigation to provide evidence that an electric current can produce a magnetic field and that a changing magnetic field can produce an electric current. [Assessment Boundary: Assessment is limited to designing and conducting investigations with provided materials and tools.] HS-PS2-6. Communicate scientific and technical information about why the molecular-level structure is important in the functioning of designed materials.* [Clarification Statement: Emphasis is on the attractive and repulsive forces that determine the functioning of the material. Examples could include why electrically conductive materials are often made of metal, flexible but durable materials are made up of long chained molecules, and pharmaceuticals are designed to interact with specific receptors.] [Assessment Boundary: Assessment is limited to provided molecular structures of specific designed materials.] 6 HS-PS3 Energy HS-PS3 Energy Students who demonstrate understanding can: HS-PS3-1. Create a computational model to calculate the change in the energy of one component in a system when the change in energy of the other component(s) and energy flows in and out of the system are known. [Clarification Statement: Emphasis is on explaining the meaning of mathematical expressions used in the model.] [Assessment Boundary: Assessment is limited to basic algebraic expressions or computations; to systems of two or three components; and to thermal energy, kinetic energy, and/or the energies in gravitational, magnetic, or electric fields.] HS-PS3-2. Develop and use models to illustrate that energy at the macroscopic scale can be accounted for as either motions of particles or energy stored in fields. [Clarification Statement: Examples of phenomena at the macroscopic scale could include the conversion of kinetic energy to thermal energy, the energy stored due to position of an object above the earth, and the energy stored between two electrically-charged plates. Examples of models could include diagrams, drawings, descriptions, and computer simulations.] HS-PS3-3. Design, build, and refine a device that works within given constraints to convert one form of energy into another form of energy.* [Clarification Statement: Emphasis is on both qualitative and quantitative evaluations of devices. Examples of devices could include Rube Goldberg devices, wind turbines, solar cells, solar ovens, and generators. Examples of constraints could include use of renewable energy forms and efficiency.] [Assessment Boundary: Assessment for quantitative evaluations is limited to total output for a given input. Assessment is limited to devices constructed with materials provided to students.] HS-PS3-4. Plan and conduct an investigation to provide evidence that the transfer of thermal energy when two components of different temperature are combined within a closed system results in a more uniform energy distribution among the components in the system (second law of thermodynamics). [Clarification Statement: Emphasis is on analyzing data from student investigations and using mathematical thinking to describe the energy changes both quantitatively and conceptually. Examples of investigations could include mixing liquids at different initial temperatures or adding objects at different temperatures to water.] [Assessment Boundary: Assessment is limited to investigations based on materials and tools provided to students.] HS-PS3-5. Develop and use a model of two objects interacting through electric or magnetic fields to illustrate the forces between objects and the changes in energy of the objects due to the interaction. [Clarification Statement: Examples of models could include drawings, diagrams, and texts, such as drawings of what happens when two charges of opposite polarity are near each other, including an explanation of how the change in energy of the objects is related to the change in energy of the field.] [Assessment Boundary: Assessment is limited to systems containing two objects.] 7 HS-PS4 Waves and Their Applications in technologies for Information Transfer HS-PS4 Waves and Their Applications in Technologies for Information Transfer Students who demonstrate understanding can: HS-PS4-1. Use mathematical representations to support a claim regarding relationships among the frequency, wavelength, and speed of waves traveling in various media. [Clarification Statement: Examples of data could include electromagnetic radiation traveling in a vacuum and glass, sound waves traveling through air and water, and seismic waves traveling through the Earth.] [Assessment Boundary: Assessment is limited to algebraic relationships and describing those relationships qualitatively.] HS-PS4-2. Evaluate questions about the advantages of using a digital transmission and storage of information. [Clarification Statement: Examples of advantages could include that digital information is stable because it can be stored reliably in computer memory, transferred easily, and copied and shared rapidly. Disadvantages could include issues of easy deletion, security, and theft.] HS-PS4-3. Evaluate the claims, evidence, and reasoning behind the idea that electromagnetic radiation can be described either by a wave model or a particle model, and that for some situations one model is more useful than the other. [Clarification Statement: Emphasis is on how the experimental evidence supports the claim and how a theory is generally modified in light of new evidence. Examples of a phenomenon could include resonance, interference, diffraction, and photoelectric effect.] [Assessment Boundary: Assessment does not include using quantum theory.] HS-PS4-4. Evaluate the validity and reliability of claims in published materials of the effects that different frequencies of electromagnetic radiation have when absorbed by matter. [Clarification Statement: Emphasis is on the idea that different frequencies of light have different energies, and the damage to living tissue from electromagnetic radiation depends on the energy of the radiation. Examples of published materials could include trade books, magazines, web resources, videos, and other passages that may reflect bias.] [Assessment Boundary: Assessment is limited to qualitative descriptions.] HS-PS4-5. Communicate technical information about how some technological devices use the principles of wave behavior and wave interactions with matter to transmit and capture information and energy.* [Clarification Statement: Examples could include solar cells capturing light and converting it to electricity; medical imaging; and communications technology.] [Assessment Boundary: Assessments are limited to qualitative information. Assessments do not include band theory.] 8 HS_ETS1 Engineering Design HS-ETS1 Engineering Design Students who demonstrate understanding can: HS-ETS1-1. Analyze a major global challenge to specify qualitative and quantitative criteria and constraints for solutions that account for societal needs and wants. HS-ETS1-2. Design a solution to a complex real-world problem by breaking it down into smaller, more manageable problems that can be solved through engineering. HS-ETS1-3. Evaluate a solution to a complex real-world problem based on prioritized criteria and trade-offs that account for a range of constraints, including cost, safety, and reliability, and aesthetics, as well as possible social, cultural, and environmental impacts. HS-ETS1-4. Use a computer simulation to model the impact of proposed solutions to a complex real-world problem with numerous criteria and constraints on interactions within and between systems relevant to the problem. 9