Acid rain is defined as Air pollution produced when acid chemicals
advertisement

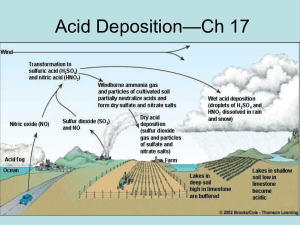
Acid rain is defined as Air pollution produced when acid chemicals are incorporated into rain, snow, fog or mist. The "acid" in acid rain comes from sulfur oxides and nitrogen oxides, these are products of burning coal and other fuels and from certain industrial processes. The sulfur oxides and nitrogen oxides are related to two strong acids: sulfuric acid and nitric acid. When sulfur dioxide and nitrogen oxides are released from power plants and other sources, winds blow them far from their source. If the acid chemicals in the air are blown into areas where the weather is wet, the acids can fall to Earth in the rain, snow, fog or mist. In areas where the weather is dry, the acid chemicals may become incorporated into dusts or smokes. Acid rain can damage the environment. human health and property. Acid rain is measured Acid rain is measured using a scale called “pH.” The lower a substance's pH, the more acidic it is.You can reduce acid rain by: Clean up smokestacks and exhaust pipes Almost all of the electricity that powers modern life comes from burning fossil fuels such as coal, natural gas, and oil. Acid deposition is caused by two pollutants that are released into the atmosphere when fossil fuels are burned: sulfur dioxide (SO2) and nitrogen oxides (NOx). Coal accounts for most U.S. SO2 emissions and a large portion of NOx emissions. Sulfur is present in coal as an impurity, and it reacts with air when the coal is burned to form SO2. In contrast, NOx is formed when any fossil fuel is burned. There are several options for reducing SO2 emissions, including using coal containing less sulfur, washing the coal, and using devices called “scrubbers” to chemically remove the SO2 from the gases leaving the smokestack. Power plants can also switch fuels—for example, burning natural gas creates much less SO2 than burning coal. Use alternative energy sources There are other sources of electricity besides fossil fuels. They include nuclear power, hydropower, wind energy, geothermal energy, and solar energy. Nuclear and hydropower are used most widely in the United States, while wind, solar, and geothermal energy have not yet been harnessed on a large enough scale to make them economically-feasible alternatives. Restore a damaged environment However, there are some things that people can do to bring back lakes and streams more quickly. Limestone or lime (a naturally occurring basic compound) can be added to acidic lakes to “cancel out” the acidity. This process, called liming, has been used extensively in Norway and Sweden but is not used very often in the United States Liming tends to be expensive, has to be done repeatedly to keep the water from returning to its acidic condition, and is considered a short-term remedy in only specific areas, rather than an effort to reduce or prevent pollution. Furthermore, it does not solve the broader problems of changes in soil chemistry and forest health in the watershed, and it does nothing to address visibility reductions, materials damage, and risk to human health. However, liming does often permit fish to remain in a lake, allowing the native population to survive in place until emissions reductions reduce the amount of acid deposition in the area. The average pH of rainfall in New York State ranges from 4.0 to 4.5, which is up to 30 times more acidic than "normal." Satisfaction of our energy needs via the combustion of fossil fuels (coal, oil, natural gas, wood, etc.) is the basic cause of acid rain. Emissions from motor vehicles, power plants and industries all contribute to acid rain. Emissions of SO2 and NOx from the heavily industrialized Midwest have been identified as significant contributors to New York State's deteriorating air quality as well. New York State has worked hard to reduce air emissions which contribute to acidic deposition, including passage of the first Acid Deposition Control Act in the nation in 1984. The New York State Legislature realized then that the state could not solve the acidic deposition problem by itself, due to the significant impact of air emissions originating primarily in the Midwest. DEC reported in its final Environmental Impact Statement on the Sulfur Deposition Control Program that 83 percent of the sulfur deposition that occurred in the southwestern Adirondacks originated outside of New York State.