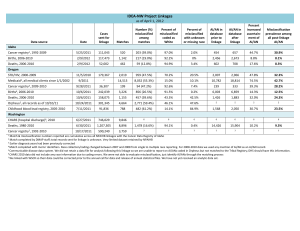
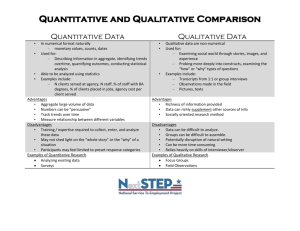
Document H. Substudy Expression of Interest Form Updated June
advertisement

Document H June 2015 Office use only EoI no: _______ Date received: ______________ Substudy Expression of Interest Use this form if you are proposing to conduct a substudy with ALSWH participants. Have you discussed this with the ALSWH Deputy Director and Research team? Yes No All substudies must be discussed with the ALSWH Research team at the University of Newcastle before filling out this form. Our substudy policy can be found in Document C at: http://www.alswh.org.au/accessingdata.html Section A Name, title, email address and institution of Project Leader (lead person) Corresponding ALSWH liaison & contact details http://www.alswh.org.au/who-is-involved/alswhliaison (If ALSWH liaison is also a collaborator please list below) Other Collaborators (include title, email address and institution) Detailed scientific title of potential project Is the research the project of a student? (will the project contribute to their award)? Yes If yes what percentage will this project contribute to their final award? (For example if their project is based entirely on ALSWH data this would be 100%) No % If yes, please provide student’s name, course, institution, course start date and expected completion date Please list supervisors Does this project involve linked data? If yes, please select the appropriate data set. The Australian Institute of Health and Welfare is the custodian for the cause of death data, which comes from the National Death Index. All researchers who use the cause of death data must provide a public interest statement for each project undertaken using COD URF data. The statement must establish a clear link between the intended use of the data for the project and the benefits to the community. Analyses on external linked datasets may only be conducted at the University of Queensland, the University of Newcastle or through the SURE facility Principal Supervisor: Associate Supervisor/s: Yes No Cancer Perinatal Admitted Patients Data Collections Aged Care MBS PBS Cause of Death If you intend to use Cause of Death data, please add this statement in this box. ☐The researcher/s will conduct analyses on site at either the University of Queensland or the University of Newcastle. This option is dependent on the availability of Document H June 2015 at the Sax Institute https://www.saxinstitute.org.au/our-work/sure For external researchers wishing to include external linked data in analyses, please select one of the following options: facilities and resources at the time of request. ☐ALSWH will provide an experienced statistician to conduct analyses on behalf of the researcher. This option will involve a cost recovery which will be negotiated on a case by case basis, based on the complexity of work involved. It is also dependent on the availability of resources. ☐Researchers will use the SURE facility. If this option is chosen, researchers are responsible for any costs imposed by SURE to access the data. Does this new EOI supersede a current or previous EOI? Yes No If yes, please provide details of which EOI it supersedes and confirm whether the EOI it supersedes can now be made inactive Please provide a lay synopsis of your proposed project (75-100 words) that can be published on the ALSWH website if the project is approved. (This synopsis may also be included in the ALSWH annual newsletter to participants.) Outline of substudy: Rationale (scientific and/or policy driven) Hypothesis/research questions, Methods (description of study type- interview, survey etc; sample size; criteria for selection/exclusion; length of survey, interview), Main variables – outcome response/dependent variables and explanatory/predictor/independent variables, Main method of analysis. Please attach drafts of any surveys/interviews you wish to use If you are also planning to analyse other ALSWH data as part of the proposed project, please specify hypotheses/research questions, main variables (as above) and analyses to be undertaken and specify the ALSWH datasets that are required. Please note – For data linkage projects the additional Variable Checklist must be completed. Ethical considerations: Participant confidentiality & care Institutions where clearance will be sought (must include Universities of Newcastle & Queensland) Status of ethics applications Expected outcomes & likely target audience (eg. paper to be submitted to a psychology journal; paper to be submitted to National Rural health Conference) Timeline for the project Develop with assistance of ALSWH UN staff Include: Start date Ethics clearance dates Recruiting start date Expected conclusion of data collection Expected outcomes date (eg. paper submission date) How do you expect to fund this work? (Describe source and amount) Budget (Develop with assistance of ALSWH staff) Document H June 2015 Core ALSWH staff who will assist & the tasks agreed upon (Develop with assistance of ALSWH staff) Names and email addresses of all people who will have access to the raw data for analysis purposes For projects using data from external datasets: Names and email addresses of all people who will have access to the linked data. All investigators accessing external datasets are required to be approved by the data custodians and HRECs where appropriate. Who will provide the substantive expertise and input? Who will provide statistical expertise and input? Who will provide qualitative analysis expertise if required? Keywords Applicable themes – tick all that apply Tobacco, alcohol & other drugs Chronic conditions Weight nutrition & physical activity Health service use & systems including Medicare Australia analyses Mental health Social factors in health & wellbeing Medications Health in rural and remote areas Formal & informal work patterns & work-family balance Roles & relationships Caring Intergenerational issues Reproductive health Methodology Data Linkage Ageing Independent living / Aged Care Abuse Please complete section B of this document on the following page Document H June 2015 Section B - ALSWH Dataset Instructions (for linked data – see Section C) Collaborator name & email address: ALSWH Liaison person for the project: The data sets on this form are specific to data collected by ALSWH. Access to external linked data sets such as Perinatal Data, Cancer registry, Admitted Patients Data Collections or MBS and PBS should be discussed with your ALSWH Liaison person. A separate form is required for use for all external linked data sets. Please see Section C for links to the appropriate variable checklist forms for all external linked data sets. Please indicate preferences for the following by checking the applicable boxes: SAS SPSS STATA Other (text) Type of data files When datasets are requested the accompanying formats and labels will also be sent if possible. Cohort 1989-95 (NYC): Survey data Survey data only Survey & qualitative data Qualitative data only 1973-78 (Young): Survey data only Survey & qualitative data Qualitative data only 1946-51 (Mid): Survey data only Survey & qualitative data Qualitative data only 1921-26 (Old): Survey data only Survey & qualitative data Qualitative data only OTHER DATA SETS 1 2 1 2 3 4 5 6 Child Data Child Data Child Data 1 2 3 4 5 6 7 1 2 3 4 5 6 7 1973-78 1946-51 1921-26 Participant Status Cause of Death 5th Survey 6th Survey 5th Survey 6th Survey Medications Food Frequency Questionnaire 1973-78 Survey 3 Survey 3 Survey 5 Survey 7 Comments: EOI No: Date EOI approved: __ /__ /___ Dataset created by: Date dataset created: __ /__ /___ 1946-51 Office use only Email address 4th Survey Document H June 2015 Section C – Requesting Linked Datasets Access to external linked data sets such as Perinatal Data, Cancer Registry, Admitted Patients Data Collections, Aged Care, MBS and PBS should first be discussed with the ALSWH Liaison person. Currently, the ALSWH has approval to link survey data with external Cancer Registry, Perinatal and Admitted Patients Data from NSW, WA, QLD, SA and VIC with annual updates. Applications are currently in progress to receive similar data from all other States and Territories. Aged Care, MBS and PBS data are currently available up to the most recent year for all participants, except for women who had previously requested to be removed. Analyses on external linked datasets may only be conducted at the University of Queensland, the University of Newcastle or through the SURE facility at the Sax Institute https://www.saxinstitute.org.au/our-work/sure. External researchers wishing to include external linked data in analyses must select one of the available options in Section A of this document. There are a number of caveats that need to be noted by any researcher using these linked data. The ALSWH will not release all the linked data for each project. The user must specify which records and specific variables they require for each age cohort. Justification for the inclusion of each variable in your study will be required. The release of variables will not be considered unless sufficient justification has been provided for each variable requested. Please see the appropriate variable checklist form and the ALSWH website for further information about the accessing data policy www.alswh.org.au. Please check the applicable boxes below and then follow the links to complete the appropriate additional variable request forms. Please attach all relevant forms to your application. ☐ ☐ ☐ ☐ ☐ ☐ ☐ ☐ Linked Data Set MBS PBS Aged Care NSW- Perinatal data, Cancer Registry, Hospital morbidity WA - Perinatal data, Cancer Registry, Hospital morbidity QLD - Perinatal data, Cancer Registry, Hospital morbidity SA - Cancer Registry, Separations (Hospital) VIC - Cancer Registry If ‘Yes’ please complete and append ALSWH Data Linkage Variable Request form Currently not available If ‘Yes’ please complete and append ALSWH Data Linkage Variable Request form If ‘Yes’ please complete and append ALSWH Data Linkage Variable Request form If ‘Yes’ please complete and append ALSWH Data Linkage Variable Request form