pbi12032-sup-0001-FiguresS1-S7-TableS1
advertisement
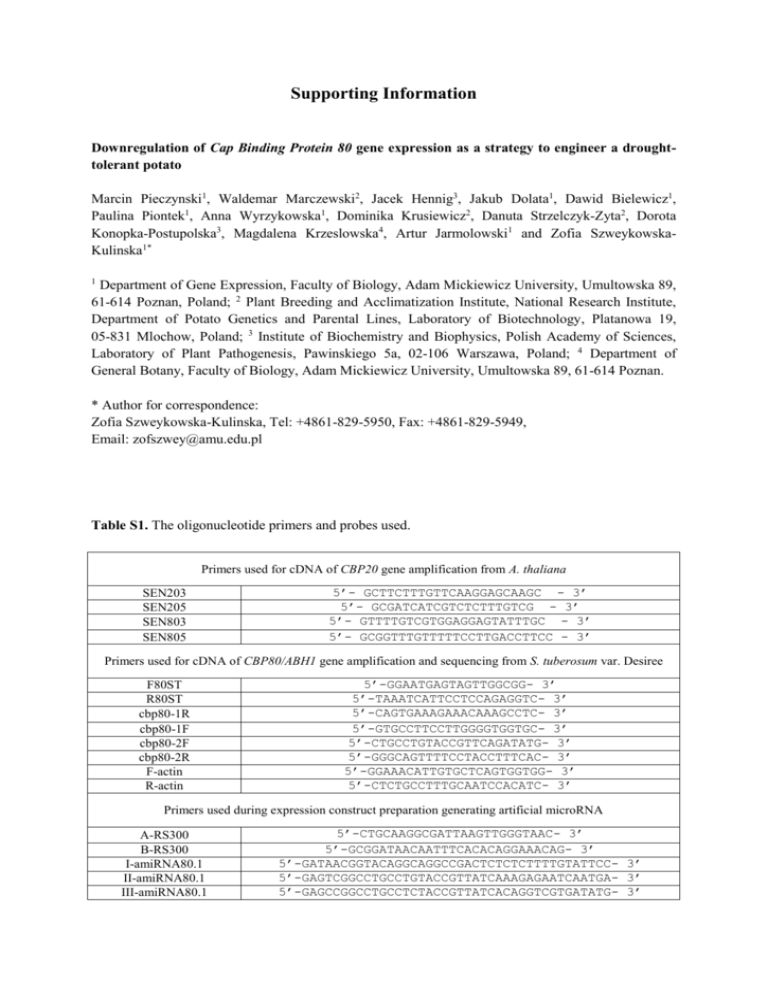
Supporting Information Downregulation of Cap Binding Protein 80 gene expression as a strategy to engineer a droughttolerant potato Marcin Pieczynski1, Waldemar Marczewski2, Jacek Hennig3, Jakub Dolata1, Dawid Bielewicz1, Paulina Piontek1, Anna Wyrzykowska1, Dominika Krusiewicz2, Danuta Strzelczyk-Zyta2, Dorota Konopka-Postupolska3, Magdalena Krzeslowska4, Artur Jarmolowski1 and Zofia SzweykowskaKulinska1* 1 Department of Gene Expression, Faculty of Biology, Adam Mickiewicz University, Umultowska 89, 61-614 Poznan, Poland; 2 Plant Breeding and Acclimatization Institute, National Research Institute, Department of Potato Genetics and Parental Lines, Laboratory of Biotechnology, Platanowa 19, 05-831 Mlochow, Poland; 3 Institute of Biochemistry and Biophysics, Polish Academy of Sciences, Laboratory of Plant Pathogenesis, Pawinskiego 5a, 02-106 Warszawa, Poland; 4 Department of General Botany, Faculty of Biology, Adam Mickiewicz University, Umultowska 89, 61-614 Poznan. * Author for correspondence: Zofia Szweykowska-Kulinska, Tel: +4861-829-5950, Fax: +4861-829-5949, Email: zofszwey@amu.edu.pl Table S1. The oligonucleotide primers and probes used. Primers used for cDNA of CBP20 gene amplification from A. thaliana SEN203 SEN205 SEN803 SEN805 5’- GCTTCTTTGTTCAAGGAGCAAGC - 3’ 5’- GCGATCATCGTCTCTTTGTCG - 3’ 5’- GTTTTGTCGTGGAGGAGTATTTGC - 3’ 5’- GCGGTTTGTTTTTCCTTGACCTTCC - 3’ Primers used for cDNA of CBP80/ABH1 gene amplification and sequencing from S. tuberosum var. Desiree F80ST R80ST cbp80-1R cbp80-1F cbp80-2F cbp80-2R F-actin R-actin 5’-GGAATGAGTAGTTGGCGG- 3’ 5’-TAAATCATTCCTCCAGAGGTC- 3’ 5’-CAGTGAAAGAAACAAAGCCTC- 3’ 5’-GTGCCTTCCTTGGGGTGGTGC- 3’ 5’-CTGCCTGTACCGTTCAGATATG- 3’ 5’-GGGCAGTTTTCCTACCTTTCAC- 3’ 5’-GGAAACATTGTGCTCAGTGGTGG- 3’ 5’-CTCTGCCTTTGCAATCCACATC- 3’ Primers used during expression construct preparation generating artificial microRNA A-RS300 B-RS300 I-amiRNA80.1 II-amiRNA80.1 III-amiRNA80.1 5’-CTGCAAGGCGATTAAGTTGGGTAAC- 3’ 5’-GCGGATAACAATTTCACACAGGAAACAG- 3’ 5’-GATAACGGTACAGGCAGGCCGACTCTCTCTTTTGTATTCC- 3’ 5’-GAGTCGGCCTGCCTGTACCGTTATCAAAGAGAATCAATGA- 3’ 5’-GAGCCGGCCTGCCTCTACCGTTATCACAGGTCGTGATATG- 3’ 5’-GATAACGGTAGAGGCAGGCCGGCTCTACATATATATTCCT- 3’ IV-amiRNA80.1 5’-GATAACGGTACAGGCAGCCGGACTCTCTCTTTTGTATTCC- 3’ I-amiRNA80.2 5’-GAGTCCGGCTGCCTGTACCGTTATCAAAGAGAATCAATGA- 3’ II-amiRNA80.2 5’-GAGTACGGCTGCCTGAACCGTTTTCACAGGTCGTGATATG- 3’ III-amiRNA80.2 5’-GAAAACGGTTCAGGCAGCCGTACTCTACATATATATTCCT- 3’ IV-amiRNA80.2 Primers used for cDNA fragments amplification of CBP80/ABH1 and cyclofilin genes from S. tuberosum during real-time PCR reactions 5’-TCCTTCAAATAAAACTGAGGATC- 3’ Fcbp80 5’-CCTGGCAGAGCCTTGC- 3’ Rcbp80 5’-CTCTTCGCCGATACCACTCC- 3’ F_cyclofilin 5’-TCACACGGTGGAAGGTTGAG- 3’ R_cyclofilin 5’-GCCCGGGTAATCTTTGAAAT- 3’ F-18S rRNA 5’-GTACAAAGGGCAGGGACGTA- 3’ R-18S rRNA Oligonucleotides used as probes during northern hybridization probe-80.1 probe-80.2 microRNA 159 U6 snRNA 5’-GTCGGCCTGCCTGTACCGTTA5’-GTCCGGCTGCCTGTACCGTTA5’- AAATGCTCCCTTTAATCCAAA 5’- TCATCCTTGCGCAGGGGCCAG 3’ 3’ -3’ -3’ Primers used for cDNA fragment amplification of RAB18 gene (At5g66400) 5’- GAAACCCGATCCAGCAGCAG -3’ RAB-F 5’- TCTTGTCCATCATCCCCTTCT -3’ RAB-R Primers used for cDNA fragments amplification of different genes from A.thaliana and S.tuberosum during real-time PCR reactions 5’- AGCATGGTGAGGGTAACTGG- 3’ AtMYB33-F 5’- TTGGCCTCAGATGATTAGCC- 3’ AtMYB33-R 5’- GGGAGAGGGTTGAAGAAAGG- 3’ AtMYB101-F 5’- CACCGTAGACGGCAACTTTT- 3’ AtMYB101-R 5’- CTCTTCCTCTGGGGTGAATG- 3’ StMYB33-F 5’- TTCAGGACTTGCTCGTTGTG- 3’ StMYB33-R 5’- TACTGGATCAATGCCCATCC- 3’ StMYB101-F 5’- GCTTGCCTGGAAGAACAGAC- 3’ StMYB101-R 5’-GCCCGGGTAATCTTTGAAAT- 3’ F-18S rRNA 5’-GTACAAAGGGCAGGGACGTA- 3’ R-18S rRNA Figure S1. A comparison of the A.thaliana T-DNA insertion and posttranscriptionally silenced cbp20 and cbp80 mutants. (a) The same minor morphological changes are present in both the posttranscriptionally silenced cbp20 and cbp80 mutants and T-DNA knockouts. (b) Both classes of mutants exhibited hypersensitivity to ABA during germination. (c) The phenotype that shows a reduction in wilting during drought stress was obtained in cbp20 and cbp80 mutants using insertion mutagenesis and posttranscriptional gene silencing. Plants are shown after 6 days without watering. Plants were grown in a growth chamber for five weeks under normal conditions before completely ceasing irrigation. Figure S2. The potato CBP80 full-length cDNA from the Desiree cultivar (line 3) was analyzed on a 1% agarose gel. Line 2 – actin (Accession number: DQ252512.1), lines 1 and 4 – 100 bp and 1 kbp DNA ladder, respectively. Figure S3. A comparison of the cDNA sequences of the CBP80 gene alleles from the potato cultivar Desiree. The black color depicts the nucleotide differences between the sequences. The red and violet colors depict the START and STOP codons, respectively. The green color depicts the sequences chosen as targets for the artificial micro RNA. Figure S4. An analysis of the CBP80 amino acid (aa) sequence from the Desiree plants. When compared with the A.thaliana CBP80 protein using the ClustalW2 program, a 67% identity and 83% similarity of the aa sequences were found. Desiree-1, Desiree-2, and Desiree-3 represent the aa sequences of individual alleles of the CBP80 protein found in the cultivar Desiree. The green, blue, and red frames depict domains from MIF4G, and amino acid letters with a red background represent the aa substitutions found in the three CBP80 alleles of the cultivar Desiree. The amino acid letters with a black background represent the aa substitutions found in the A.thaliana and potato CBP80 proteins. Figure S5. The level of silencing of CBP80 gene expression using artificial miRNAs in selected potato plants. Individual plants from the two potato transgenic lines amiR80.1-8 and amiR80.2-14 were tested for the silencing of CBP80 gene expression during the third year of vegetative reproduction via tubers. Real-time measurements of the level of CBP80 mRNA in selected transgenic plants were calculated as a percentage of the expression of CBP80 mRNA in the Desiree plants. As a comparison, the level of silencing from one individual plant in 2010 is shown. Levels of transcript were normalized against cyclophilin. Values are shown as the mean ±SD (n=3) from three independent experiments. Asterisk - P<0.02, Mann-Whitney U-Test. Figure S6. Quantitative real-time PCR of the Arabidopsis pri-mir319a in wild-type and cbp80 mutant plants. The levels of transcript were normalized against GAPDH (At1g13440). The calculation shows the mean ±SD from three biological replicates. Figure S7. Real-time measurements of the levels of the StMYB1R-1 mRNA using the ΔΔCT method in the Desiree and amiRNA80.2-14 plants after 14 days without watering. The mRNA level is presented as a percentage of the StMYB1R-1 mRNA level in the control plants at day 0 of drought. Levels of transcript were normalized against cyclophilin.