Name Ph of a common substance lab Background: Liquids all
advertisement

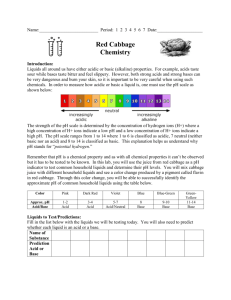
Name __________________________________________________________________Date_______________ Ph of a common substance lab Background: Liquids all around us have either acidic or basic (alkaline) properties. In our Homeostasis: if the blood becomes a bit acidic or a bit basic, then a person becomes sick. For homeostasis to be regained, the person breathes quicker to rid the body of carbon dioxide. This lab will teach you what it means to say something is acidic, basic or neutral. Let’s explore this. Vocabulary: a) ion – an atom with a charge b) dissociate – a substance that breaks into ions Water is a substance. It is written as H2O. It can dissociate: H2O H+ + OH- . The H+ is called the hydrogen ion and the OH- is called the hydroxide ion. Study the beaker diagram. It is filled with water. The water has dissociated. Count the number of H+ in the beaker. How many are there? _______ Count the number of OH- in the beaker? _______ What do you notice about the number of H+ and OH-? ___________________________ Let’s take that same beaker of water and add an acid like lemon juice to make an acidic liquid. Study the beaker below. Count the number of H+ in the beaker. How many are there? _______ Count the number of OH- in the beaker? _______ What do you notice about the number of H+ and OH- after adding the lemon juice? __________________________________________. Let’s get another beaker of water and add a base like bleach to it to make a basic liquid. Study the beaker below. Count the number of H+ in the beaker. How many are there? _______ Count the number of OH- in the beaker? _______ What has happened to the number of H+ and OH- after adding the bleach? ____________________________________________ Let’s think about what we just examined happening in each of the beakers. See if you can answer these questions to help you understand if a liquid is an acid, a base or neutral. 1. Water had equal amount of ______ and _____. Substances like water are called neutral. 2. Acidic liquids have ________ H+ than OH-. Acids, like lemon juice, add __________ ions. 3. Basic liquids have _________ H+ than OH-. Bases, like bleach, add _________ ions. 4. The more _______ added to a liquid, the stronger the acid it becomes. Time to do the Lab! Question: Which common liquids are acids, bases or neutral? To determine if a liquid is acidic or basic, one needs to measure the amount of H+ ions. The use of pH paper determines the amount of hydrogen ions (H+). When the paper is wet with the liquid, it will change colors. The color will indicate if the solution is acidic, neutral or basic. Basic The numbers on the pH scale indicates the concentration of hydrogen ions (H+) in a solution. Solutions with a pH below 7 are acidic. The lower the number, the more H+ and the stronger acid it is. Liquids with a pH above 7 are basic. The higher the number, the more OH- and the stronger the base it is. Bases have even less H+ than acids. A pH of 7 represents a neutral solution, one that is neither an acid nor a base. Pure water has a pH of 7. Pre-lab Questions: 1. Why must you use clean glassware in experiments involving pH? 2. What does pH measure? 3. What are buffers? 4. Is a pH of a strong base higher or lower than the pH of a weak base? _________________________ 5. Is the pH of a strong acid higher or lower than the pH of a weak acid? ________________________ 6. Name the products of a neutralization reaction. Materials: Test tubes with unknown liquids and test tube rack Stirring rod pH paper Procedure: 1. 2. 3. 4. 5. Obtain the liquids in the test tubes in the test tube rack. Obtain a 250ml beaker with stirring rods. Fill half the beaker with water from the tap. Obtain a container of pH paper. Examine the color and number scale on the side of the container. Using a pipette, fill 2 mL of each liquid substance in six different test tubes. Obtain a piece of paper toweling. Place 6 strips of the pH paper on the towel equally spaced apart. Underneath the pH strip, write the name of the solution. 6. Take your stirring rod and dip it into the first liquid to be tested. 7. Blot the stirring rod on the pH paper. Observe the paper and note the color of the paper. Compare the color of the pH paper to the color scale on the pH paper container. Record the pH color, the pH number of the liquid, and if it is an acid, base, or neutral substance in the data table. 8. Dip the stirring rod into a beaker of water to rinse off testing liquid, and dry off with a paper towel. 9. Repeat steps 1-6 for each of the other liquids you are testing. BE SURE TO RINSE OFF THE STIRRING ROD IN BETWEEN EACH TEST SO THAT YOU DON’T CONTAMINATE THE OTHER LIQUIDS!!!! 10. Throw out pH paper and paper toweling in trash. Clean out all test tubes. Return all materials to the place you got them from and answer the questions. Data: * Use the data table below to record your observations. Refer back to the table to help you answer the conclusion questions. Liquid Color of Blue Litmus Color of Red Litmus pH number Acid, Base, or Neural Paper Paper 1. Orange Juice 2. Vinegar 3. Milk 4. Egg White 5. Baking Soda 6. Ammonia Water Analysis: Categorize your results below: Strong Acids Weak Acids Neutral Weak Base Strong Base 1. Pick two different liquids. List the two liquids that you tested below, and Explain how the number of hydrogen ions compare in each. 2. What would happen to the orange juice if you were able to add more hydrogen ions? Explain 3. Your stomach makes a very strong acid, called hydrochloric acid (HCl). This is good because the stomach needs to have a pH of 1.5-3 when food is present. Sometimes people make acid when there is no food present which keeps the stomach fluids too acidic. Heartburn, ulcers, stomach irritation and discomfort occur because the acid damages the lining of the stomach. a) If a person suffers from any of the symptoms listed above, are they in homeostasis? Explain. b) Tums or Maalox are two medications that can help make the person feel better temporarily. These medications neutralize the acid and make the stomach fluids less acidic. What is happening to the pH of the stomach when a person takes the medication, and how is this happening? Explain.