properties
advertisement

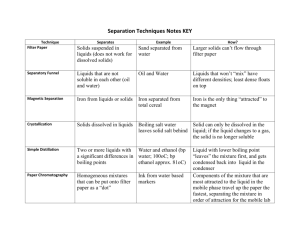
Background for the unit: There are different states of matter and each of these states has properties that are unique. What are the different states of matter and what properties do they possess that allows us to classify them? Do items exist that possess qualities of more than one state of matter? Matter is the stuff from which tangible objects are made. This includes pencils, mountains, and oceans. There are three types of matter that we are likely to encounter in a typical day. They are: solids, liquids and gasses. Solid objects have definite shape and the shape doesn’t change when the object is moved from one place to another. When solids are moved from one container to another, they maintain the same shape as they had before. Solids that have been reduced to very small particles, like sand, have some of the same characteristics as liquids. Powdered materials pour and spill and they appear to fill containers to a level. However, powders do not form flat surfaces and can be made into piles and pushed into shapes on flat surfaces. Liquids have no shape of their own, but do have a constant volume. Liquids take the shape of their containers and fill it to level. The surface of a liquid is always flat and level with the plane of the earth. Gasses have no shape or constant volume. Gasses expand and contract readily. Gasses are difficult to handle and are hard to interact with. Some materials are composed of solids and liquids. These materials have some properties of solids and some of liquids. These can be described as a new state of matter - colloid. Interactions between solids, liquids and solids and liquids produce a variety of results. Mixtures of solids can be separated into their components, even though it can be a challenge at times. Mixtures of liquids are interesting, they may mix completely, they may react to produce new products or they may not mix and will form layers. Mixtures of solids and liquids can produce all of the above results plus some others. The solid may sink or float, it might soak up the liquid, and it might dissolve in the liquid. Due to the difficulties in dealing with gasses, this unit will focus only on solids and liquids, but the students will be informed of some properties of gasses. Minnesota State and NSES Standards: Physical Science: Structure of Matter: The students will understand that objects can be sorted and classified based on their properties. History and Nature of Science: A Scientific Inquiry: The students will raise questions about the natural world, make careful observations and seek answers. What will students KNOW? Materials can be sorted based on their properties. Properties of solids, including: flexible, rigid, smooth, rough, soft, hard, colored, pointed, flat, transparent, opaque Properties of liquids: transparent, translucent, viscous, free flowing, foamy, clear, liquids take the shape of their containers, and liquids pour and flow. Some solids behave like liquids. All matter takes up space and has mass. What will students UNDERSTAND? The students will understand that matter has three states. The students will understand that objects can be classified based on their properties. The students will understand that matter can be changed. What will students be able to DO? The students will sort and classify objects in terms of color, size, shape, weight, texture, and flexibility. The students will classify a substance as a solid, liquid or gas. The students will know that solids have a definite shape and that liquids take the shape of their container. The students will observe that water can be a solid or liquid and can change from one state to another. The students will use appropriate tools to gather and organize data. The students will recognize and describe patterns The students will describe changes in matter Guiding Questions: Core Curriculum: What is a solid? What are the properties of solids? What is a liquid? What are the properties of liquids? What happens when solids are mixed? Liquids are mixed? Solids and liquids are mixed? What are two properties that all matter has? What are some ways in which matter changes? What are some causes of the changes? Curriculum of Practice: What types of tools are needed to sort and classify solids and liquids? How do scientists use their knowledge of the types of matter and their properties? What types of job utilize information about solids and liquids? Curriculum of Connections: Where do you see uses of solids and liquids in your community? How have our buildings changed over time as we better understand solids and liquids? How does our knowledge of solids and liquids change our views of pollution and environmental issues? How are solids and liquids recycled and reused? What are two helpful changes in matter? Two harmful changes in matter? Curriculum of Identity: How does my knowledge of solids and liquids help me to construct a building? How does my knowledge of solids and liquids change my views of recycling and reusing? Can you name two ways in which changing matter affects you? Materials and Resources FOSS kit for solids and liquids Heath Science book Chapter 3 Unit on Solids and Liquids United Streaming Videos on Solids and Liquids BrainPop videos on states of matter EdHelper.Com Worksheets National Geographic Video *Because there is so much core knowledge to learn in this unit, the early parts are mainly structured inquiry. Engagement Stage – Building Solids: Pass out bags filled with solid materials: cloth square, plastic triangle, metal screw, wooden cylinder, rubber band, straw, craft stick, rubber tube, aluminum foil, Dixie cup, and a piece of coated wire. Allow the students to explore the objects looking for similarities and differences. Ask students to describe these objects. Teacher will have to introduce the term properties to the class. Properties are a way to describe an object. Exploration Stage – Building Solids Teacher challenges the students to use the materials to build the tallest free-standing structure they can. Before they can begin exploring, have the students predict which materials would work best. They begin investigating individually and then using the knowledge they gained, work as a team. Allow enough time for the groups to share their towers and what materials work best for constructing a tower. Students should journal how each material works to construct. Then pose the question, “Would those same materials be best for building a long, low structure – like a bridge for instance?” Explanation Stage – Building Solids After students have had time to explore experiment and analyze each of the materials for construction. Communicate successes and frustrations and what learning took place. Elaboration Stage – Building Solids Have students move from using the small solids that they have in the classroom to using the knowledge they gained and apply it to real world situations. Engagement Stage – Beans and Beads This requires that students move to each of the six stations, observing the same thing with each of the solids: lima beans, pinto beans, rock salt, corn meal, rice and mung beans. At each station have the students observe how the materials pour and pile. The students should also make observations about how the solids move and feel. Exploration Stage – Beans and Beads Provide each group of students bottles ½ filled with each of the following items: lima beans, mung beans, pinto beans, rice, and cornmeal. Have the students explore how the bottles roll, spin, travel down an incline plane, shake, and tip. Students should record their observations and any questions they have onto a graphic organizer of their own design. Students will mix the solids together and then separate them using funnels, screens and tubs. Explanation Stage – Beans and Beads Compare and contrast the different solids, their properties and how they behave. Communicate their knowledge to the class. Elaboration Stage – Beans and Beads Generate a list of questions that could be explored in the classroom environment using the solids and tools we have available. Students may also bring in other solids with the teacher’s approval. Exploration Stage – Liquids Pass out several different sized and shaped containers to each group of students. Have them pour 25 ml of water into each container and observe what happens. Students should discuss their observations and questions. Teacher will have to introduce the term level to the class at this time and provide the students with information about how water acts as a level. (Bring in a level from home if one is available). Engagement Stage – Liquids Give each group of students bottles of the following liquids: fabric softener, hand soap, liquid detergent, corn syrup, colored water, water, and cooking oil. Allow the students to explore how each of the liquids behaves when the bottle is tipped, rolled, spun, and shaken. Students should collect data on a graphic organizer of their own design. Teacher will have to introduce different property words: viscous, foamy, and bubbly. Explanation Stage – Liquids Have students analyze all the different liquids and communicate their learning to the rest of the class. Elaboration Stage – Liquids and Solids Have students generate a list of questions to investigate about liquids. They may create plans and conduct investigations individually or in small groups. They are only limited to using the materials that are available in class. (Do not have them bring in new liquids because of possible reactions when mixed). * At this time, encourage students to generate questions involving both the solids and the liquids. They may mix solids with solids, solids with liquids, and liquids with liquids. Have students conduct investigations and communicate their learning with the rest of the class. Possible investigations include: evaporation, condensation, and mixtures. Once students have had a chance to conduct their own investigations, pose the questions, “What is toothpaste, a liquid or a solid? How do you know?” Allow the students to create investigations to determine the answer. Teacher will have to introduce colloid as the investigations conducted in class will yield mixed results for toothpaste. This is a great time to show the BrainPop movie on states of matter. Engagement Stage – Gas Teacher prepares 2 empty water bottles for this ‘experiment’. The first bottle (the one the teacher will use) has small holes poked in the bottom. The second (the student bottle) remains unchanged. Place a balloon into each bottle, stretching the mouth of the balloon over the mouth of the bottle. Bring one student to the front to see if a balloon can be blown up inside a bottle. Because air takes up space, the bottle with no holes will not allow the balloon to expand, but the teacher’s bottle will allow the balloon to expand since the air can escape from the bottle. Exploration Stage – Gas Have the students examine the two balloons and bottles – making sure no one else tries to blow them up. You may want to give each child a balloon to try in each bottle (have several prepared so groups can be exploring at the same time). Have the students record and discuss their observations. If no one begins to “blame the bottle” the teacher may have to direct them to finding that difference. Students can tell air is escaping from one bottle by holding their hand below it. Explanation Stage – Gas Have students share findings with the rest of the class. *At this time if no one has discovered it, the teacher can show how to blow up the balloon in the student bottle by squeezing the bottle. The balloon will come out the top of the bottle and will be filled with the air from inside the bottle. Elaboration Stage – Gas Have students generate a list of questions to investigate about gasses. Teacher should demonstrate the Bernoulli principal by blowing between two filled balloons to show how the air pressure brings them together. Students can re-create this with a piece of paper folded into a tent – they blow through the tent and the sides come together before the tent collapses. Teacher can also show how we know air takes up space and has mass by weighing an empty balloon and a blown-up balloon. Teacher may also demonstrate for the class how gas created from vinegar and baking soda can fill up a balloon. That same gas can be used to blow out a candle in a jar. Assessments: Student journals, conference notes taken during group work about student attitudes and effort, pre and post test about matter, student investigation reports, self assessment, reflective writing from the students about how they’ve grown as scientists, investigators and in their knowledge of the states of matter.