SUPPLEMENTARY MATERIAL Antibacterial activity of essential oils
advertisement

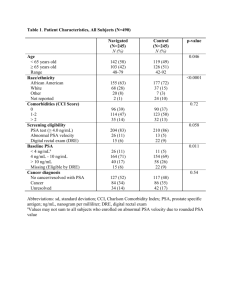
SUPPLEMENTARY MATERIAL Antibacterial activity of essential oils mixture against PSA. Elisabetta Vavalaa, Claudio Passarielloa, Federico Pepib, Marisa Colonec, Stefania Garzolib, Rino Ragnod, Adele Pirollid, Annarita Stringaroc, Letizia Angiolellaa*. a Department of Public Health and Infectious Diseases. University of Rome "Sapienza", Piazzale Aldo Moro, 5, 00161 Roma, Italy. b Department of Drugs Chemistry and Technology, University of Rome "Sapienza",Piazzale Aldo Moro, 5, 00161 Roma, Italy. c Department of Technology and Health, Italian National Institute of Health. Viale Regina Elena 299, 00161 Rome, Italy. d Rome Center for Molecular Design, Department of Drugs Chemistry and Technology, University of Rome "Sapienza", Italy *Corresponding author. Mailing address: Department of Public Health and Infectious Diseases. University of Rome "Sapienza". Piazzale Aldo Moro, 00161-Rome (Italy). Phone: +3964468625, Fax: +3964468625. E-mail: letizia.angiolella@uniroma1.it 1 Abstract Pseudomonas syringae pv actinidiae (PSA) is the causal agent of bacterial canker of kiwifruit. It is very difficult to treat pandemic disease. The prolonged treatment with cupric substances, has resulted in failure and resistance and alternatives to conventional antimicrobial therapy are needed. The aim of our study was to analyse the phenotypic characteristics of PSA, identify new substances from natural source i.e essential oils able to contain the kiwifruit canker and investigate their potential use when utilised in combination. Specially, we investigated the morphological differences of PSA isolates by scanning electron microscope (SEM), and the synergic action of different essential oils by time-kill and checkerboard methods. Our results demonstrated that PSA was able to produce extracellular polysaccharides when it was isolated from trunk, and, for the first time, that it was possible to kill PSA with a mixture of essential oils after 1 h of exposition. We hypothesise on its potential use in agriculture. Key words: Pseudomonas syryngae pv actinidiae, kiwifruit canker, exopolymeric material, SEM, Essential oils, synergism. 3. Experimental 3.1 Microorganism and growth conditions. Strains of Pseudomonas syryngae pv actinidiae (PSA) were obtained from a case of bacterial canker on A. chinensis in central Italy (Latina province) in 2011. Bacteria were isolated from trunk and from exudates and were identified by the techniques described below. The strains were cultured on nutrient agar (Oxoid) supplemented with 5% sucrose (NSA) and incubated at 25–27°C or room temperature for 24 h. 3.2 Exopolysaccharides Production. The exopolysaccharides production (EPS) was evaluated by streaking fresh culture of bacteria isolated from trunk or exudate on NSA agar supplemented with 5% (w/v) sucrose (Sigma). After incubation at 25-27°C under anaerobic conditions for 72 h, the development of a mucoid colony on agar medium indicated the production of exopolysaccharides (Ruas-Madiedo & de los ReyesGavilan 2005). 3.3 Molecular identification of PSA. Cultures of both strains for DNA extraction were grown overnight in Luria Bertani Broth (LB) at 26°C on a rotating shaker at a speed of 150 rpm. DNA was extracted using the DNeasy Kit 2 (Qiagen). DNA concentration was determined spectrophotometrically at 260 nm, assuming that an OD=1 corresponded to a DNA concentration of 50 ng/l. For amplification of PSA specific DNA fragments of the 16S–23S rDNA inter transcribed spacer region was used as the PCR protocol of Rees-George (Rees-George et TTTTGCTTTGCACACCCGATTTT, al. 2010). PsaR2 Reverse (Primers: PsaF1 Forward CACGCACCCTTCAATCAGGATG amplifying a 280 bp specific fragment). The PCR reaction mixture (25 l final volume) contained 10 ng template DNA, primers at 0.5 M each, dNTPs at 200 M each, 0.5 U DreamTaq DNA polymerase (Fermentas), Dream Taq DNA polymerase buffer containing 20 mM MgCl2. Amplification was obtained by an initial denaturation at 95°C for 2 min, followed by 30 cycles at 95°C for 30 s (denaturing), 65°C for 30 s (annealing) and 72°C for 30 s (elongation). Final elongation was at 72°C for 5 min. PCR amplification products were detected by ethidium bromide staining, following separation on 2% agarose gel in Tris Acetate EDTA buffer. The 1kb DNA Ladder (Fermentas) was used as the molecular weight internal standard. 3.4 Scanning electron microscope (SEM). For SEM observations, bacteria were grown in5% glucose-supplemented NA medium (see above) at 25-27 °C for 24h. After being washed twice in calcium and magnesium-free PBS, the cellular pellets resulting from the centrifugation were fixed for 20 minutes at room temperature with 2.5% (v/v) glutaraldehyde in a 0.2M cacodylate buffer (pH 7.4) containing 2% (w/v) sucrose. After three washes in the same buffer, the cells were post-fixed with 1% (w/v) OsO4 for 1 h, dehydrated on an ethanol gradient, critical point dried in CO2 and sputter bv coated with gold (Stringaro et al. 2014). The samples were examined under a Cambridge Stereoscan 360 scanning electron microscope (Cambridge Instruments, Cambridge, UK). 3.5 Extraction and chemical characterization of Essential oils. Mentha suaveolens essential oil was obtained from areal part of wild-type plants recovered in the Tarquinia contry-side (Viterbo province) as previously described (Angiolella et al. 2010) (EOMS patent WO 2011092655 A3) Rosmarinus officinalis (REO), Salvia officinalis (SEO) and Laurus nobilis (LEO) were obtained from leaves of wild-type plants in Poggio Moiano country-side (Rieti province) by four-hour hydro distillation (Clevenger-type apparatus) and then analysed for chemical composition by GC/MS analyses (Perkin Elmer Clarus 500) and GC-FID equipped with a Restek Stabilwax fused-silica capillary column (60 m long, 0.25 mm i.d.), operating at 60°C for 5 min and then programmed at 220°C rate of 5°C/min.; helium was used as carrier gas with 1 ml/min. Relative weight percentages of the components were calculated by electronic integration of the GC-FID peak areas and were identified by comparison of their GC-MS mass spectra with those reported in the 3 NIST and NBS libraries. Compounds were identified by the application of the NIST 08 Mass Spectral Library. Analysis, as reported in table S 2, revealed that 90% of EOMS was constituted by piperitenone oxide (Angiolella et al. 2010). REO was constituted principally by 20.3 % borneole, 13.0 % alpha pinene, 11.4 % camphor, 8 % eucalyptol and by other minor constituents. The main chemical components of SEO were 25.8% caryophillene, 22.4% caryophyllene 16.5% eucalyptol and other minor constituents. 18.1% camphene,16.9% cynnamil acetate and 15% eucalyptol represent the main components of LEO. The essential oil of Melaleuca alternifolia (TTO) used in this study was kindly gifted by Talia (Rome, Italy, http:/ www.taliaessenze.com). It was constituted principally by 53.8 % terpinen 4-olo, 14.3 % gamma terpinene and 9.6 % alpha terpineol. 3.6 Antimicrobial assays. The susceptibility of the microbial cells to EOs and ampicillin sodium salt, used as a control (Sigma -Aldrich), was determined by broth microdilution assays of the Clinical and Laboratory Standards Institute (CLSI 2009) with inoculum of approximately 105colony forming units per milliliter (CFU/ml) of each microorganisms. The EOs were 1:10 diluted in DMSO and subsequently diluted in water. The cellular growth was observed after 24h. The dilutions of the EOs, ranging from 0.01219 to 12.48 g/L, were prepared in a 96-well plate. The minimum inhibitory concentration (MIC) was defined as the lowest concentration of essential oils without visible growth. To determine the Minimum Bactericidal Concentration (MBC), NSA dextrose agar plates were seeded with 10μl cell suspensions taken from the wells of the MIC assay plates where cell growth was not observed. These plates were incubated at 25-30°C for 48h and CFU growth was evaluated. 3.7 EOs synergism To evaluate synergism between different essential oils,three-dimensional chequerboard assay was used (Bhusal et al. 2005). To test and optimise synergistic combinations, serial dilution of the three oils (REO, TTO and EOMS) was used in a 96-well plate starting from twice the MIC of the individual antimicrobial agents. The presence and extent of synergism was evaluated by calculating the fractional inhibitory concentration index (FICI) as the combined EO effect of agents A,B and C (where A is EOMS,B is TTO and C is REO) and was calculated as follows: Fractional inhibitory concentration index (FICI) = FIC A + FICB +FICC = A/MICA + B/MICB+ C/MICc where A,B and C are the MICs of each EO in combination and MIC A, MICB and MICC are the MICs of each EO alone. The results of the FICI assay were analysed according to the checkerboard method (Rej-Jurado et al. 2013) adapted to three drugs in combination. In this case the synergism was defined when FICI was lower or equal to 0.75; indifferent was determined from 0.75 to 4; and a FICI> 4 was considered as antagonistic activity. 4 3.8 In vitro time-kill synergy kinetics. Time-kill curve methods were used to evaluate the synergistic activities of EOs in combination with each other against PSA. An overnight broth culture of PSA was diluted in NSA broth to obtain a starting inoculum of about 1 × 105 CFU/ml. Essential oils in combination were added to the bacterial broth at different concentrations of synergy values (1/2x, 1x, 2x synergy values). 100μl aliquots of broth were taken after 0, 30, 60, 120, 360 min and 24 h of incubation. Each aliquot was serially diluted, plated onto NSA agar plates in duplicates and incubated overnight at 25-27°C. The number of colony forming units was counted and CFU/ml was determined. The values were plotted to obtain the kill-kinetics. Results are expressed as CFU/ml. 5 Figure S1 .Isolation and molecular identification of PSA. A) Top view of spot colonies of PSA, at 2 days of incubation on nutrient agar supplemented with 5% sucrose (NSA),using a 10x objective. B) PCR reactions to identify PSA. Line 1 represents DNA marker, lines 2 and 3 DNA extracted by strains isolated both from trunk and exudate. 6 Table S2. GC-FID chemical composition of Essential Oils (weight %) 7 Figure S2. Time-kill curves of EOMS, REO and TTO against PSA. Bacteria were untreated (control) or treated with mixture of EOs at concentrations equivalent to ½, 1, or 2 times their synergic values (s.v.) for different time periods(0, 30 min, 1,2, 6 and 24 h). Data are representative of one of three independent experiments. The statistical significance was evaluated with the Student’s t-test (two-tailed). *P-values of < 0.05 were considered significant References Angiolella, L., Vavala, E., Sivric, S., Diodata, D’A. F., & Ragno, R. (2010). In vitro activity of Mentha suaveolens essential oil against Cryptococcus neoformans and dermatophytes. International Journal of Essential Oil Therapeutics, 4: 35-36. Bhusal. Y., Shiohira, C.M., & Yamane, N. (2005) Determination of in vitro synergy when three antimicrobial agents are combined againstMycobacterium tuberculosis.International Journal of Antimicrobial Agents, 26: 292-297. Clinical and Laboratory Standards Institute. (2009). Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically. 8th ed. Wayne: CLSI Rey-Jurado, E., Tudo, G., de la Bellacasa, J.P., & Espasa, M. (2013). In vitro effect of three drug combinations of antitubercular agents against multidrug–resistant Mycobacterium tuberculosis isolates. International Journal of Antimicrobial Agents, 41: 278-280. Ruas-Madiedo P., & de los Reyes-Gavilán, C.G. (2005). Invited Review: Methods for the Screening, Isolation, and Characterization of Exopolysaccharides Produced by Lactic Acid Bacteria. Journal of Dairy Science, 88: 843-856 Stringaro, A., Vavala, E., Colone, M., Pepi, F., Mignogna, G., GarzoliS.,Cecchetti, S., Ragno, R., & Angiolella, L. (2014). Effects of Mentha suaveolens essential oil alone or in combination with other drugs in Candida albicans. Evidence-Based Complementary and Alternative Medicine, 2014, 9 pages, article ID125904 . 8