Physiology Ch. 36 p451-461 [3-25
advertisement

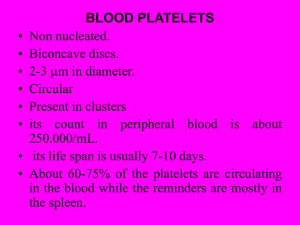
Physiology Ch. 36 p451-461 Hemostasis and Blood Coagulation Hemostasis – prevention of blood loss, such as when a vessel is ruptured -achieved by vascular constriction formation of platelet plug formation of blood clot growth of fibrous tissue into clot to close the hole Vascular Constriction – a cut of rupture to vessel causes smooth muscle in vessel to contract to reduce flow of blood from this vessel. -contraction results from 3 things: local myogenic spasm (most of the contraction), local autacoid factors, nerve response -platelets are responsible for much of the vasoconstriction by releasing thromboxane A2 Formation of Platelet Plug – small cuts in vessels sealed by platelet plug and do not form a clot Platelets – thrombocytes, minute discs 1-4um in diameter and formed from megakaryocytes -normal concentration of platelets in blood is 150,000 – 300000/mL -Do not have nuclei and do not reproduce, but they contain: 1. Actin + myosin – contractile proteins as well as thrombosthenin, causing platelet contraction 2. Residual ER and golgi – synthesize enzymes and store calcium 3. Mitochondria – synthesize ATP and ADP 4. Enzymes that synthesize prostaglandins – hormones to cause vascular and tissue reactions 5. Fibrin-stabilizing factor – related to blood coagulation 6. Growth factor to cause vascular endothelial cells, smooth muscle and fibroblasts to proliferate -cell membrane of platelets have glycoproteins that repulses adherence to endothelium and causes adherence to injured areas on vessel wall -cell membrane also contains lots of phospholipids that activate multiple stages of clotting -platelet lives for 8-12 days and is eliminated by tissue macrophages mainly in spleen Mechanism of Platelet Plug – platelets change when in contact with damaged vascular surface, especially with collagen fibers -they swell and protrude pseudopods they contract and release granules containing active factors -become sticky and adhere to collagen and von Willebrand factor that leaks into tissue from plasma and secrete ADP -their enzymes form thromboxane A2, and ADP + Thromboxane act to activate other platelets to cause them to become sticky, which causes them to adhere to other platelets -adherent platelets cause a platelet plug – first a loose plug, but is usually successful in blocking blood loss, then during coagulation, fibrin threads form and attach to platelets to reinforce them -platelet-plugging mechanism is extremely important for closing minute ruptures in small blood vessels that can occur daily Blood Coagulation – blood clotting is the third mechanism for hemostasis, and begins to develop in 1520 seconds to severe vascular trauma -activator molecules from vascular wall, platelets, and blood proteins adhering to damaged wall initiate clotting process Vessel is severed Platelets agglutinate fibrin appears fibrin clot forms clot retraction occurs Fibrous Organization or Dissolution of Blood Clot – Once clot has formed, it can either be invaded by fibroblasts to form connective tissue throughout clot, or ot can dissolve -usual route for clotting is invasion of small hole in vessel wall by fibroblasts promoted by growth factor and completes in 1-2 weeks -If too much blood has leaked into tissues where not needed, clots tend to dissolve Basic Theory of Blood Coagulation – procoagulants promote coagulation while anticoagulants inhibit coagulation -whether blood will coagulate depends on the balance of these substances -anticoagulants normally most active in blood unless in local tissue injury where procoagulants active General Mechanism of Coagulation – clotting takes place in 3 steps 1. Cascade of reactions take place in blood to form prothrombin activator in response to injury 2. Prothrombin activator converts prothrombin to thrombin 3. Thrombin is an enzyme that converts fibrinogen fibrin that enmesh platelets and RBC for clot Conversion of Prothrombin to Thrombin – prothrombin activator is formed as a result of vessel rupture -in the presence of Ca2+, prothrombin activator converts prothrombin to thrombin -thrombin causes polymerization of fibrinogen molecules to fibrin dibers in 10-15 seconds -rate limiting factor is production of prothrombin activator -prothrombin FIRST attaches to prothrombin receptors on platelets in damaged tissue Prothrombin and Thrombin – prothrombin is an alpha2-globulin in plasma at a conc. Of 15mg/dL -unstable and can split to thrombin -continually made by LIVER and is used in body for blood clotting -Vitamin K is required by liver for activation of prothrombin + other clotting factors Conversion of Fibrinogen to Fibrin – Fibrinogen is a big protein in plasma with conc. Of 100-700mg/dL -fibrinogen is formed in liver and normally leaks out from blood vessels into interstitial fluids (no coagulation here) -If capillary is damaged, fibrinogen will leak into tissue fluids in sufficient quantities to allow clotting Action of Thrombin on Fibrinogen Fibrin – thrombin is an enzyme with proteolytic activity -acts on fibrinogen to remove 4 low molecular weight fibrin monomers from the polypeptide -Fibrin monomers immediately polymerize into fibers within seconds to form reticulum of clot -in early polymerization, fibrin monomers are weakly held together by H bonds and not cross-linked -initial clot is weak and can be broken apart -A fibrin-stabilizing factor strengthens the fibrin reticulum after being released from platelets and plasma -Thrombin has to activate fibrin-stabilizing factor in order for it to form covalent bonds between fibrin monomers Blood Clot – clot is composed of a mesh of fibrin fibers in all directions entrapping RBC, platelets and plasma. Fibrin fibers adhere to damaged surfaces of vessels and any vascular opening to prevent loss Clot Retraction – Serum – after a few minutes of clot formation, it contracts and expresses fluid in 20-60 minutes. -Fluid is called serum because all its fibrinogen and clotting factors are removed -Platelets necessary for retraction to occur (failure of retraction might = low platelets) -platelets attached to fibers change fibers completely, and continue to release procoagulant factors such as fibrin-stabilizing factor -platelets help the clot by activating thrombosthenin, actin, and myosin to contract and reduce size -as clot retracts, edges of broken blood vessel pulled together to contribute to further hemostasis Positive Feedback of Clot Formation – once a clot has started, It initiates a positive feedback to promote more clotting, such as thrombin acting on many clotting factors including fibrinogen -blood clot will continue to grow until leakage ceases Initiation of Coagulation: Formation of Prothrombin Activator – prothrombin activator is formed upon trauma to vascular wall, trauma to blood, and contact of blood with damaged endothelial cells. -Prothrombin activator is formed in either: 1. Extrinsic Pathway – begins with trauma to vascular wall and surrounding tissues 2. Intrinsic Pathway – begins in the blood itself -in both pathways, blood-clotting factors play major roles as proteolytic enzymes that cause cascades when activated -Most clotting factors listed as roman numerals, and have an “a” after them to indicate active form Extrinsic Pathway for Initiating Clotting – begins with traumatized vascular wall or extravascular tissue 1. Release of tissue factor also called tissue thromboplastin composed of phospholipids from tissue + lipoprotein complex that functions as proteolytic enzyme 2. Lipoprotein complex of tissue factor complexes with Factor VII (Makes Factor VIIa) and Calcium to activate Factor X to Factor Xa 3. Factor Xa combines with tissue phospholipids in tissue factors and Factor V to form prothrombin activator a. Prothrombin activator splits prothrombin to thrombin and clotting proceeds b. Factor V is initially inactive in the prothrombin activating complex, but becomes active when thrombin begins to form c. Activated factor Xa is the protease that splits prothrombin into thrombin, and Factor V accelerates this activity (positive feedback) Intrinsic Pathway for Initiating Clotting – begins with trauma to blood or exposure of blood to collagen from traumatized vessel wall 1. Blood trauma activates Factor XII and release of platelet phospholipids a. Factor XII in contact with collagen takes up new configuration Factor XIIa b. Platelet touching collagen damages them to release platelet phospholipids containing platelet factor 3 2. Active factor XIIa activates Factor XI Factor XIa in presence of High molecular weight (HMW) kininogen, and is accelerated by prekallikrein 3. Active Factor XIa then enzymatically activates factor IX factor IXa 4. Active factor IXa + Factor VIIIa + platelet phospholipids + platelet factor 3 activates factor X factor Xa a. If factor VIII or platelets are missing, this step is deficient. Classic hemophilia occurs when factor VIII is missing from a person (factor VIII also called antihemophilic factor) b. Thrombocytopenia – bleeding disease in which person lacks platelets for clotting 5. Factor Xa combines with Factor V and platelet or tissue phospholipids to form prothrombin activator cleaves prothrombin to thrombin and activating fibrinogen fibrin Role of Calcium Ions in Intrinsic and Extrinsic Pathways – except for 1st two reactions, Ca ions required for all blood-clotting reactions -without calcium, blood clotting does not occur -When blood is removed from person, it can be prevented from clotting by reducing Ca ion concentration below threshold for clotting with citrate ion or oxalate ion -Both pathways happen simultaneously – where tissue factor stimulates extrinsic pathway and Fact XII and platelets with collagen in vascular wall initiate intrinsic pathway -Extrinsic pathway is explosive, limited only by amount of tissue factor and by quantity of factor X, VII, and V -Intrinsic pathway is much slower 1-6 minutes Prevention of Blood Clotting -> Intravascular Anticoagulants – 1. Endothelial Surface Factors – most important factors for preventing clotting normally are: a. the smoothness of endothelial cell surface to prevent contact activation b. layer of glycocalyx on endothelium which repels clotting factors and platelets c. protein bound on endothelial membrane called thrombomodulin binds thrombin i. complex of thrombomodulin + thrombin activates protein C that acts as anticoagulant by binding to Factors V and VIII -if endothelial wall is damaged, glycocalyx-thrombomodulin layer is lost, and activates factor XII and platelets 2. Antithrombin Action of Fibrin and Antrithrombin III – most important anticoagulants in blood are those that remove thrombin a. Fibrin fibers – formed during clotting process and absorb 90-95% of thrombin to help prevent its spread in the blood b. Antithrombin III – thrombin not absorbed by fibrin blocks thrombin’s effect on fibrinogen and inactivates thrombin 3. Heparin – powerful anticoagulant that is in low concentration in blood. Used as pharmacological agent in medical practice to prevent intravascular clotting a. Heparin is negatively charged polysaccharide that combines with antithrombin III which increases its effectiveness 100x b. Heparin complexed with antithrombin III also removes Factors XII, XI, X, and IX c. Produced by many cells, especially basophilic mast cells in pericapillary connective tissue throughout body Lysis of Blood Clots – Plasmin – plasma proteins conatain a euglobulin called plasminogen that becomes plasmin when activated -Plasmin resembles trypsin, but digests fibrin fibers and other coagulants such as fibrinogen, factor V, VIII, prothrombin, and Factor XII. -Plasmin formation can cause lysis of blood clot -When clot is formed, large quantities of plasminogen is trapped in clot with other plasma proteins -injured tissues very slowly release powerful activator called tissue plasminogen activator that converts plasminogen to plasmin after a few days once bleeding has stopped to remove the remaining unnecessary clot Conditions that Cause Excessive Bleeding in Humans – three particular types of bleeding have been studied: Vitamin K deficiency, hemophilia, and thrombocytopenia (platelet deficiency) 1. Vitamin K deficiency – liver synthesizes most of the clotting factors, and in liver diseases clotting system can get ruined a. Vitamin K is essential factor to a liver carboxylase that adds carboxyl group to glutamate on prothrombin, factors VII, IX, X, and protein C. Vit. K becomes oxidized and inactive until vitamin K epoxide reductase complex 1 reduces it back b. Without Vit. K, bleeding can occur c. Vit. K is synthesized by intestinal bacteria, and so deficiency rarely occurs as a result of absence from diet i. In GI disease, vitamin K deficiency results from poor absorption of fats from GI tract since vit. K is a fat soluble vitamin d. One of most prevalent causes of Vit. K deficiency is failure of liver to secrete bile into GI tract to provide adequate fat digestion and absorption (depresses Vit. K as well) i. Thus, liver diseases causes poor vit. K absorption and decreased prothrombin formation e. Vitamin K can be given intravenously 2. Hemophilia – bleeding disease that occurs almost exclusively in males a. Abnormality or deficiency in Factor VIII (causing hemophilia A or classic hemophilia) b. In the rest (15%) of cases, hemophilia is caused by deficiency of factor IX c. Both of these are transferred genetically by X chromosome, so that female will rarely have the disease, but she can be a carrier d. Bleeding can have various degrees of severity, but does not usually occur until trauma e. Factor VIII has 2 active components, the smaller of which is most important in the intrinsic pathway of clotting, deficiency of which causes classic hemophilia f. Von Willebrand’s Disease – results from deficiency of large component of Factor VIII g. Therapy for classic hemophilia is injection of pure factor VIII 3. Thrombocytopenia – presence of very low numbers of platelets in blood. Tendency to bleed, except bleeding is from many small venules or capillaries as opposed to hemophiliacs which bleed from larger vessels a. Small hemorrhages occur in all body tissues, and skin becomes blotchy purple, giving the name of the disease thrombocytopenic purpura b. Bleeding will not usually occur until platelet count falls below 50,000/uL c. Suspect thrombocytopenia if blood fails to retract d. Most people get idiopathic thrombocytopenia of unknown cause, where antibodies are made against platelets e. Blood transfusions and splenectomy are helpful Thromboembolic Conditions in the Human -Abnormal clot developing in vessel is called a thrombus. Continued flow past the clot is likely to break it away and cause it to travel through the blood, where it is them called an embolus (emboli). -if originating in large arteries, they can travel to brain, kidneys or lungs Cause of Thromboembolic Conditions – roughened endothelial surface of a vessel as caused by arteriosclerosis, infection or trauma that initiates clotting process. Blood clots can occur if blood flow is very slow through blood vessels where coagulants are formed (sedentary person) -genetically engineered tissue plasminogen activator can be delivered to thrombosed area to activate plasminogen into plasmin to dissolve the clot Femoral Venous Thrombosis and Massive Pulmonary Embolism – clotting occurs when blood flow is blocked for many hours in any vessel, and so immobile patients in bed can cause clotting to occur in one or more leg veins -clot can grow and move toward venous return blood in entire leg and into common iliac or vena cava veins -clot can disengage and flow through venous blood through right side of the heart and into pulmonary arteries to cause massive pulmonary embolism – leads to death of both arteries are clogged -tissue plasminogen activator delivery could be life-saving Disseminated Intravascular Coagulation – happens when clotting mechanism is activated in widespread areas of circulation, caused by large amounts of dying tissue in the body -occurs in patients with septicemia, where circulating bacteria and endotoxins activate clotting -partly the reason why septic shock is lethal in 85% of patients -peculiar effect of disseminated intravascular shock is that patient often bleeds Anticoagulants for Clinical Use – most common are heparin and coumarins 1. Heparin – causes blood clotting time to increase from normal 6 minutes to about 30 minutes. Action of heparin lasts 1.5-4 hours and is destroyed by an enzyme called heparinase 2. Coumarins – Warfarin is an example… when given to patients, amonuts of prothrombin, Factors VII, IX, and X all fall a. Warfarin – causes this by inhibiting the enzyme vitamin K epoxide reductase complex 1, which converts vitamin K to its active, reduced form. i. by inhibiting VKOR c1, warfarin decreases available active vit K in tissues, such that coagulation factors are no longer carboxylated and are inactive ii. After administration of warfarin, coagulant activity decreases to about 50% normal after 12 hours and 20% normal after 24 hours Prevention of Blood Coagulation Outside the Body – blood collected in siliconized containers does not clot for 1 hour or more, because silicone prevents platelet and Factor XII activation -heparin can be used to prevent coagulation outside the body in surgical procedures -substances to decrease Ca concentration of blood can prevent coagulation outside the body -citrate ion can prevent coagulation by deionizing calcium Blood Coagulation tests – bleeding time after sharp pointy knife pricks finger or earlobe (normal is 1-6 minutes) -Clotting time is measured by putting blood on clean glass tube and tip it until clotted (6-10 min) -Prothrombin time and international normalized ratio – prothrombin time – gives an indication of amount of prothrombin in the blood, it is the time required for coagulation to take place -international normalized ratio (INR) standardizes prothrombin time by using standardized tissue factor from manufacturer