I Love to Hate the Coagulation Cascade Katy W. Waddell, RVT, VTS
advertisement

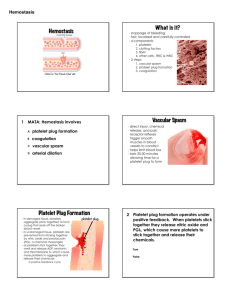
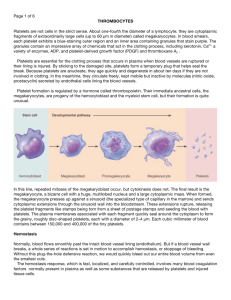
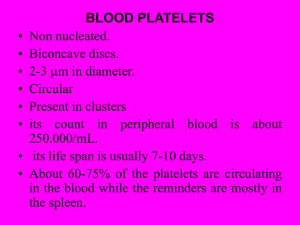
I Love to Hate the Coagulation Cascade Katy W. Waddell, RVT, VTS (ECC, Anesthesia) Hemostasis is divided into three separate categories, primary hemostasis, secondary hemostasis and tertiary hemostasis (also called fibrinolysis). Primary hemostasis is initiated when any damage to small blood vessels or capillaries occur. The immediate response to the damage is a reflex that promotes vasoconstriction which in itself can diminish or decrease blood loss. Disruption or damage to the vessel exposes the endothelial lining of the vessel. Collagen (a supportive protein) is exposed and will provide a surface for the platelets to adhere to. von Willebrand factor (vWf) binds to a glycoprotein in the platelet membrane. Platelets, when coming into contact with collagen fibers in the vascular wall or damaged endothelial cell, begin to swell and assume irregular forms. These irregular forms have many irradiating (star shaped) processes which protrude from their surfaces. These contractile proteins contract with force and cause the release of granules that contain multiple active factors. They become quite sticky and adhere to the collagen fibers. We may define primary hemostasis as the formation of the platelet plug. If the hole in the blood vessel is small, it is managed by the formation of a platelet plug rather than what we think of as a blood clot. There is a sequence of events which occurs at the site of vascular injury. First, the platelet is attracted to the exposed sub-endothelial layer of collagen and adheres to it. To ensure this, the platelet undergoes a shape change. Secondly, the platelets release intrinsic adenosine diphosphate (ADP), among other substances. The released ADP stimulates other platelets to stick together at the wound site and thirdly, aggregation occurs. In this process, platelets adhere to each other to form a beginning plug. Finally, coagulation occurs and fibrin forms around the platelet aggregate to initiate repair. The formation of the clot typically begins within 15 to 20 seconds in the case of severe vascular wall trauma and within 1 to 2 minutes if the trauma has been minor. To further this time line, 3 to 6 minutes following the rupture of a vessel the broken end of the vessel or the entire opening is filled with a clot (if the vessel opening is not too large). Within 20 minutes to one hour, retraction of the clot begins further closing the vessel. There are two separate pathways that activate the clotting cascade but these often take place at the same time. Both pathways eventually merge into the common pathway that results in the formation of stabilized fibrin. The extrinsic pathway involves a protein called tissue factor (factor III) that is found on various cells including those of the subendothelium. In damaged blood vessels, circulating factor VII works with tissue factor (factor III) and forms an active protease (VIIa) that eventually results in prothrombin being converted into the key active enzyme thrombin. The intrinsic pathway is triggered when Factor XII (Hageman Factor) comes into contact with collagen on the subendothelium of damaged blood vessels and is activated to Factor XIIa. This eventually results in prothrombin being converted into the key active enzyme thrombin. Extrinsic Pathway is also referred to as the tissue factor) pathway. The primary role of the extrinsic pathway is to create an immediate response. After damage has occurred to a blood vessel, factor II,(prothrombin) is released from circulation coming in contact with factor III Damaged tissue releases factor III (thromboplastin), which with the aid of Ca++ will activate factor VII. These form the activated complex of VII (VIIa). This initiates the extrinsic mechanism. The intrinsic pathway is the intrinsic factors that are found within the vascular space. Prekallikrein, activates factor XII. Prekallikrein is a proenzyme circulating in the plasma with high-molecular weight kininogen (HWMK). Factor XIIa converts HWMK into bradykinin. Bradykinin is an important mediator of vasodilation, inflammation and fibrinolysis. Factor XII is a plasma protein and from active platelets will activate factor XI, thus initiating the intrinsic mechanism.Both active factor VII and active factor XI will promote cascade reactions, eventually activating factor X. Active factor X (Xa), along with factor III, factor V, Ca++, and platelet thromboplastic factor (PF3), will activate prothrombin activator. The intrinsic mechanism of prothrombin activator starts with trauma or exposure of the blood to collage in a traumatized blood vessel wall. The intrinsic mechanism of prothrombin activator formation begins with trauma to the blood or exposure of blood to collagen in a traumatized vessel wall. This usually also results in damage to fragile platelets. The formation of a clot by this mechanism usually takes 1 to 6 minutes. This cascade begins with the activation of factor XII and the release of platelet factor 3 (PF3) from damaged platelets. Activated factor XII cleaves and activates factor XI and prekallikrein (PK). Factor XII is also activated by activated prekallikrein (aPK) in an internal amplification loop. Calcium (Ca++) is required for the initial three steps. The prothrombin activator in the intrinsic pathway is very similar to the activator in the extrinsic pathway. The prothrombin activator converts prothrombin to thrombin. This is an enzymatic C cleavage Thrombin is produced by the enzymatic cleavage of two sites on prothrombin by activated Factor X (Xa). The activity of factor Xa is greatly enhanced by binding to activated Factor V (Va). Thrombin, acting as a serine protease, converts the soluble fibrinogen into insoluble strands of fibrin. Fibrin initially forms a loose mesh, but then factor XIII causes the formation of covalent cross links, which convert fibrin to a dense aggregation of fibers. Platelets and red blood cells become caught in this mesh of fiber, thus the formation of a blood clot.