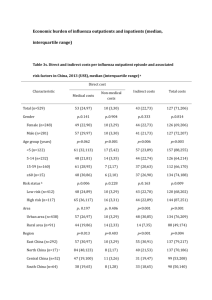
Dr. John J. Treanor, Chapter 162, Influenza Virus
advertisement

Chapter 162 – Influenza Virus JOHN J. TREANOR Influenza is an acute, usually self-limited, febrile illness caused by infection with influenza type A or B virus that occurs in outbreaks of varying severity almost every winter. The attack rates during such outbreaks may be as high as 10% to 40% over a 5- to 6-week period. The most common clinical manifestations are fever, malaise, and cough. The two most important features of influenza are the epidemic nature of the disease and the mortality that results in part from its pulmonary complications. HISTORY Influenza virus has been causing recurrent epidemics of febrile respiratory disease every 1 to 3 years for at least the past 400 years.[1][2] Although the disease is not associated with a characteristic manifestation such as rash, the high attack rate, the explosive nature of the epidemic, and the frequency of cough allow the identification of some past epidemics. For example, Sydenham’s account of an epidemic that occurred in 1679 is a clear description of influenza.[3] Hirsch tabulated 299 outbreaks occurring at an average interval of 2.4 years between 1173 and 1875.[1] As discussed later, severe epidemics of worldwide scope occur less often and are referred to as pandemics. The first recorded pandemic that clearly fits the description of influenza occurred in 1580, although others may have occurred earlier. Since then, 31 pandemics have been described. The greatest pandemic in recorded history occurred in 1918–1919 when, during three “waves” of influenza, 21 million deaths were recorded worldwide, among them 549,000 in the United States.[4] The modern understanding of influenza was ushered in by Smith and associates when they isolated influenza A virus in ferrets in 1933.[5] Influenza B virus was isolated by Francis in 1939[6] and influenza C virus by Taylor in 1950.[7] The discovery by Burnet in 1936 that influenza virus could be grown in embryonated hens’ eggs allowed extensive study of the properties of the virus and the development of inactivated vaccines.[8] Animal cell culture systems for the growth of influenza viruses were developed in the 1950s.[9] The phenomenon of hemagglutination, which was discovered by Hirst in 1941, led to simple and inexpensive methods for the measurement of virus and specific antibody.[10] Evidence of the protective efficacy of inactivated vaccines was developed in the 1940s.[11][12] Vaccines have been in widespread use in various parts of the world since, but usually for only selected segments of a population. The use of live vaccines for influenza was first suggested shortly after the virus was discovered,[13] but the first live vaccine was not licensed in the United States until 2003, approximately 70 years later. Finally, four antiviral agents in two classes have been approved for prevention and treatment of influenza. These include the so-called M2 inhibitors, amantadine in the mid-1960s, rimantadine in 1993, and the neuraminidase inhibitors zanamivir and oseltamivir in 2000. Although the M2 inhibitors are active against only influenza A, the neuraminidase inhibitors are clinically active against both influenza A and B viruses. THE VIRUSES Classification Influenza viruses belong to the family Orthomyxoviridae and are classified into three distinct types, influenza A, influenza B, and influenza C virus, on the basis of major antigenic differences. In addition, there are significant differences in genetic organization, structure, host range, epidemiology, and clinical characteristics between the three influenza virus types ( Table 162–1 ). However, all three viruses share certain features that are fundamental to their biologic behavior, including the presence of a host-cell derived envelope, envelope glycoproteins of critical importance in virus entry and egress from cells, and a segmented genome of negative sense (i.e., opposite of message sense), single-stranded RNA. The standard nomenclature for influenza viruses includes the influenza type, place of initial isolation, strain designation, and year of isolation. For example, the influenza A virus isolated by Francis[14] from a patient in Puerto Rico in 1934 is given the strain designation A/Puerto Rico/8/34, sometimes referred to as PR8 virus. Influenza A viruses are further divided into subtypes on the basis of their hemagglutinin (H) and neuraminidase (N) activity (e.g., H1N1 or H3N2). Table 162-1 -- Differences among Influenza A, B, and C Viruses Influenza A Influenza B Influenza C Genetics 8 gene segments 8 gene segments 7 gene segments Structure 10 viral proteins 11 viral proteins 9 viral proteins M2 unique NB unique HEF unique Host range Humans, swine, equine, Humans only avian, marine mammals Humans swine and Antigenic shift and Antigenic drift only. More Antigenic drift Epidemiology drift. Drift is generally than one variant may only. Multiple linear cocirculate variants Clinical features May cause large pandemics with significant mortality in young persons Severe disease generally Mild disease confined to older adults or without persons at high risk; seasonality pandemics not seen Morphologic Characteristics The morphologic characteristics of all influenza virus types, subtypes, and strains are similar. Electron microscopic studies estimate their size to be 80 to 120 nm in diameter and show them to be enveloped viruses covered with surface projections or spikes. They may exist as spherical or elongated filamentous particles as well ( Fig. 162–1 ). The latter predominate in newly isolated strains, whereas most laboratory-adapted strains consist almost entirely of spherical particles. The filamentous forms vary in length but may be up to 40 nm long. A schematic diagram of an influenza A virus is shown in Figure 162–2 . Figure 162-1 Electron micrograph of influenza A/USSR/77 H1N1 (×189,000). Figure 162-2 Schematic model of an influenza A virus. Eight structural proteins have been identified in influenza A viruses. The surface spikes are glycoproteins that possess either hemagglutinin (HA) or neuraminidase (NA) activity. Each rod-shaped HA spike measures approximately 4 nm in diameter by 14 nm in length. They can be removed from the intact virion by sodium dodecyl sulfate, by bromelain, or by chymotrypsin. Each spike is a trimer composed of three HA polypeptides, each with a molecular weight of 75,000 to 80,000, resulting in a trimer with a molecular weight of approximately 224,640.[15] The HA is synthesized as a monomer (HA0), which is cleaved by host-cell proteases into HA1 and HA2 components that remain linked together. Antigenic sites and sites for binding to cells are located in the globular head of the molecule. The viral NA is an enzyme that catalyzes the removal of terminal sialic acids (Nacetyl neuraminic acid) from sialic acid–containing glycoproteins. The NA spike is shaped like a mushroom rather than a rod and has a molecular weight of 240,000. The intact NA consists of a tetramer of NA polypeptides, each with a molecular weight of 58,000. As in HA, the antigenic sites and the enzyme active site are located in the mushroom-shaped head. At least 15 highly divergent, antigenically distinct HAs have been described in influenza A viruses (H1 to H15), as well as at least nine distinct NAs (N1 to N9). A third integral membrane protein, the M2 protein, is also present in small amounts on the viral envelope. Interior to the envelope is the matrix, or M1, protein.[16] This protein is believed to provide stability to the virion. Within the envelope are eight physically discrete nucleocapsid segments ( Table 162–2 ). Each nucleocapsid is composed of a single segment of genomic RNA intimately associated with the viral nucleoprotein (NP), with the three polymerase proteins PB1, PB2, and PA bound to one end. These socalled internal viral proteins are important targets for cross-reactive, viral-specific cytotoxic T lymphocytes (CTL). Two nonstructural viral proteins, NS1 and NS2 (also referred to as the nuclear export protein, or NEP), are also found within infected cells. Small amounts of NEP may be present within virions. Table 162-2 -- Genes and Protein Products of Influenza A Virus RNA Segment Number Gene Product Name of Protein Description 1 PB1 Basic polymerase RNA transcriptase 1 2 PB2 Basic polymerase Cap binding, 2 cleavage 3 PA Acidic polymerase Unknown 4 HA Hemagglutinin Viral attachment to cell membranes; membrane fusion 5 NA Neuraminidase Cleaves sialic acid from cell surface; released from membranes; prevents aggregation 6 NP Nucleoprotein Encapsidates RNA; regulation transcription/replication M Matrix Surrounds viral core; controls nuclear transport M2 Matrix 2 Ion channel; required for uncoating Nonstructural Antagonizes type I interferons, may be involved in regulation of mRNA transport from nucleus 7 8 Proposed Functions endonucleolytic of NS1 NEP (NS2) Nuclear export Transport of newly assembled RNP protein from nucleus to cytoplasm (?structural) EPIDEMIOLOGY Disease Impact Influenza epidemics are regularly associated with excess morbidity and mortality,[17] usually expressed in the form of excess rates of pneumonia and influenza-associated hospitalizations and deaths during epidemics.[17][18] Pneumonia and influenza deaths fluctuate annually in a predictable fashion with peaks in the winter and troughs in the summer. Observed pneumonia- and influenza-related death rates are compared with an expected baseline derived from a time-series regression model,[19] which allows calculation of excess mortality due to influenza epidemics. A tabulation of levels of excess pneumonia and influenza deaths (i.e., deaths in which specific International Classification of Diseases [ICD]9 codes are recorded as the cause of death) attributable to influenza epidemics[20] is shown in Table 162–3 , compared with the estimated percentage of isolates which were typed as influenza A (H3N2), A (H1N1), or B in each year. Significant levels of excess mortality are reported in most years. Generally, the level of excess mortality is highest in years when influenza A (H3N2) viruses predominate, but influenza B and to a lesser extent H1N1 viruses also can be associated with excess mortality. Because not all influenza-related deaths are manifested as pneumonia, the pneumonia and influenza mortality statistics probably underestimate the true impact of influenza on the population. Table 162–3 also lists the all-cause excess mortality, defined as deaths due to any cause, above a similarly derived baseline, that occur during periods of influenza epidemic activity. Although less precise than the pneumonia- and influenza-related deaths, all-cause mortality is probably a more accurate reflection of the total burden of influenza. Recent studies suggest that even higher levels of mortality might be attributable to influenza, potentially as high as 51,000 deaths annually in the United States.[21] Table 162-3 -- Estimated Excess Pneumonia- and Influenza-Related Deaths and Excess Mortality of All Causes during Influenza Epidemics Percent of Isolates That Were Pneumoniaand All-Cause of the Following (Sub) Type Influenza-Related Excess Excess Deaths Year H3N2 H1N1 B Deaths (Range) (Range) 1972/73 90 0 10 7900 (5500–10,300) 18,300 (1200– 35,000) 1973/74 20 0 80 00 00 1974/75 100 0 0 6500 (4100–8900) 15,100 32,100) 1975/76 70 0 30 11,800 (9200–14,400) 24,600 (3400– 45,900) 1976/77 5 0 95 00 00 1977/78 60 26 14 8300 (6000–10,500) 46,200 (19,800– 72,700) 1978/79 0 98 2 00 00 1979/80 2 1 97 5100 (3500–6700) 17,300 34,100) 1980/81 77 23 0 11,700 (9100–14,200) 47,200 (27,800– 66,600) 1981/82 1 24 75 2100 (600–3700) 00 1982/83 79 10 11 4700 (2800–6700) 9600 (0–19,200) 1983/84 5 50 45 3500 (1600–5400) 8200 (0–17,600) 1984/85 97 0 3 8100 (6600–9600) 36,200 (17,700– 54,700) 1985/86 24 0 76 6700 (4900–8500) 34,000 (6800– 61,200) 1986/87 — — — 1800 (1100–2500) 16,800 (1900– 31,700) 1987/88 0 80 20 7400 (5600–9100) 33,400 (12,900– 53,800) 1988/89 45 45 10 5100 (3600–6600) 10,500 (0– (600– (800– Year Percent of Isolates That Were Pneumoniaof the Following (Sub) Type Influenza-Related H3N2 H1N1 B Deaths (Range) and All-Cause Excess Excess Deaths (Range) 20,200) 1989/90 90 1 9 10,100 (8500–11,700) 43,600 (27,600– 59,600) 1990/91 4 3 93 4200 (2400–6100) 23,000 46,000) 1991/92 19 81 0 6600 (5600–7700) 41,700 (19,600– 63,700) (0– Data from Table 153–3 in Treaner JL. Influenza virus. In: Mandell GL, Bennett JE, Dolin R, eds. Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases, ed. 5. Philadelphia: Saunders; 2000:1826; and Simonsen L, Clarke MJ, Williamson DW, et al. The impact of influenza epidemics on mortality. Introducing a severity index. Am J Public Health. 1947;87:1944–1950. Mortality is only the most severe manifestation of influenza impact, and similar techniques can be used to estimate excess morbidity due to influenza epidemics.[22] Data from the Tecumseh Community Health Study have been used to estimate that influenza is responsible for from 13.8 to 16.0 million excess respiratory illnesses per year in the United States among individuals less than 20 years of age, and for 4.1 to 4.5 million excess illnesses in older individuals.[22] Influenza is usually associated with a U-shaped epidemic curve. Attack rates are generally highest in the young, whereas mortality is generally highest among older adults ( Fig. 162–3 ).[17][23] Excess morbidity and mortality are particularly high in those with certain high-risk medical conditions, including adults and children with cardiovascular and pulmonary conditions such as asthma, or those requiring regular medical care because of chronic metabolic disease, renal dysfunction, hemoglobinopathies, or immunodeficiency.[24] Influenza-related death rates in nursing home residents with comorbid conditions are as high as 2.8% per year.[25] Figure 162-3 Typical epidemic curve in the interpandemic era, showing the rates of medically attended illness (green line, rate per 100), hospitalizations for acute respiratory disease (blue line, rate per 10,000), and pneumonia- and influenza-related mortality (red line, rate per 100,000) by age for several seasons of influenza in Houston, Texas. Attack rates and hospitalizations occur at both extremes of age, but mortality occurs largely in those older than 65 years. (Data from Glezen WP, Keitel WA, Taber LH, et al. Age distribution of patients with medically attended illnesses caused by sequential variants of influenza A/H1N1: Comparison to age-specific infection rates, 1978–1989. Am J Epidemiol. 1991;133:296–304.) Influenza also results in more severe disease and significant mortality in individuals with human immunodeficiency virus (HIV) infection,[26][27] in those with iatrogenic immunosuppression,[28] and in women in the second or third trimester of pregnancy.[29] Influenza is being increasingly recognized as an important health problem in young children. Rates of influenza-related hospitalizations are particularly high in healthy children under 2 years of age, where rates approach those of older children with high-risk conditions.[30][31][32] In addition, a high rate of secondary complications, particularly otitis media and pneumonia, occur in children with influenza infection.[33] Disease impact is particularly severe in both adults and children with chronic pulmonary diseases, especially asthma.[34][35][36] It should be recognized that although complication rates are higher in older adults and debilitated persons, the majority of individuals hospitalized during influenza epidemics were ambulatory and leading productive lives prior to their acute illness.[24] Much of the impact of influenza is related to the malaise and consequent disability that it produces, even in young, healthy individuals. It has been estimated that a typical case of influenza, on average, is associated with 5 to 6 days of restricted activity, 3 to 4 days of bed disability, and about 3 days lost from work or school.[37][38] The average number of medical visits for cases in which medical attention was sought was from 1.1 to 3.6, depending on year of the outbreak and age of the patient. It is worth noting that direct medical costs of illness account for only about 20% or the total expenses of a case of influenza, with a major proportion (30% to 50%) of the economic impact due to loss of productivity. In one study, influenza in schoolchildren resulted in 37 missed school days by children and 20 days of missed work by parents, per 100 children.[39] Influenza is also associated with decreased job performance in working adults[40][41] and reduced levels of independent functioning in older adults.[42] Epidemic Influenza An epidemic is an outbreak of influenza confined to one location, such as a city, town, or country. In a given community, epidemics of influenza A virus infection have a characteristic pattern. A graphic description of an epidemic due to an A/Victoria/75/H3N2-like virus that occurred in 1976 in Houston, Texas, is shown in Figure 162–4 . Such localized epidemics begin rather abruptly, reach a sharp peak in 2 to 3 weeks, and last 5 to 6 weeks.[43] Reports of increased numbers of children with febrile respiratory illness are often the first indication of influenza, although on occasion, an outbreak in a nursing home is the very first indication of influenza in a community. Outbreaks in children are usually soon followed by the occurrence of influenza-like illnesses among adults. The next event is increased hospital admissions for patients with pneumonia, exacerbation of chronic obstructive pulmonary disease, croup, and congestive heart failure. Increased school and industrial absenteeism also occurs, but these events are insensitive and late indicators of influenza in a community.[43] Although an increased number of deaths due to pneumonia is a highly specific indicator of influenza, it invariably lags behind the other indications because of two factors: the time from the onset of illness to time of death and the delay involved in reporting deaths to public health officials.[44] During epidemics, average overall attack rates are estimated to be 10% to 20%, but in selected populations or age groups, attack rates of 40% to 50% are not unusual.[45] The factors that lead to termination of an outbreak in any given location are unclear, because usually the outbreak ceases before the supply of susceptible individuals is exhausted. Figure 162-4 Correlation of the nonvirologic indexes of epidemiologic influenza with the number of isolates of A/Victoria virus according to week, Houston, 1976. (Industrial absenteeism is determined by the percentage with respiratory complaints.) (From Glezen WP, Couch RB. Interpandemic influenza in the Houston area, 1974–1976. N Engl J Med. 1978;298:587–593, with permission.) In temperate climates in either hemisphere, epidemics occur almost exclusively in the winter months (generally October to April in the Northern Hemisphere, and May to September in the Southern Hemisphere), whereas influenza may be seen year round in the tropics. The reasons for these seasonal changes are not entirely clear. Modeling studies suggest that the effect can mostly be explained by postulating seasonal effects on virus transmissibility.[46] Such effects could be the result of more favorable environmental conditions for virus survival,[47] or of behavioral changes that increase transmission, such as indoor crowding. In large countries such as the United States or Australia, regional differences in the time occurrence of influenza outbreaks are also apparent. It is not uncommon to have major outbreaks occurring in some communities or regions while others are experiencing modest activity or none whatsoever. Usually, a single strain of influenza virus will prevail during an epidemic of influenza, and other respiratory viruses decrease in frequency.[45][48] However, this is not always the case, and occasionally two different strains within a single subtype (e.g., A/Victoria/3/75/H3N2 and A/Texas/1/77/H3N2)[49] or two different influenza A subtypes (H1N1 and H3N2) circulate simultaneously. Furthermore, concomitant outbreaks of influenza A and B or simultaneous outbreaks of influenza A and respiratory syncytial virus have been demonstrated.[50] In many years, the end of the influenza epidemic season is characterized by a brief spike in cases due to a new strain. These limited outbreaks, which have been referred to as a “herald wave,” often predict the predominant strain in the next influenza season.[51] Pandemic Influenza In contrast to the familiar pattern of epidemic influenza, pandemics are severe outbreaks that rapidly progress to involve all parts of the world and are associated with the emergence of a new virus to which the overall population possesses no immunity. Characteristics of pandemics include extremely rapid transmission with concurrent outbreaks throughout the globe; the occurrence of disease outside the usual seasonality, including during the summer months; high attack rates in all age groups, with high levels of mortality particularly in healthy young adults[52]; and multiple waves of disease immediately before and after the main outbreak. The interval between pandemics is quite variable and unpredictable, but it is likely that pandemics of influenza will continue to occur in the future. Antigenic Variation One of the unique and most remarkable features of influenza virus is the frequency with which changes in antigenicity occur; these changes are referred to as antigenic variation. Alteration of the antigen structure of the virus leads to infection with variants to which little or no resistance is present in the population at risk. The phenomenon of antigenic variation helps explain why influenza continues to be a major epidemic disease of humans. Antigenic variation involves principally the two external glycoproteins of the virus, HA and NA, and is referred to as antigenic drift or antigenic shift, depending on whether the variation is small or great. Antigenic Drift Antigenic drift refers to relatively minor antigenic changes that occur frequently (every year or every few years) within the HA and/or NA of the virus. It is generally accepted that the mechanism of antigenic drift, which has been studied more intensively for the HA, is one of gradual accumulation of amino acid changes in one or more of the five identified major antigenic sites on the HA molecule.[53] Because antibody generated by exposure to previous strains does not neutralize the antigenic variant as effectively as it did the wild type, immunologic selection takes place, and the variant supplants previous strains as the predominant virus in the epidemic. Support for this thesis comes from experimental work demonstrating that antigenic variants (generated by drift) can be produced in cell cultures in the presence of limiting amounts of antibody, and these variants have similar single amino acid sequences in the HA.[54][55][56][57] Comparison of the HA gene sequences of influenza viruses isolated in successive years reveals differences in the patterns of HA evolution between influenza A, B, and C viruses. Generally, a single lineage, or relatively few lineages, of influenza A virus circulate in humans, and the accumulation of point mutations in the HA is linear, with each strain replacing the previously circulating one. This is particularly true of H3 influenza A viruses.[53][58] In contrast, multiple lineages of influenza C virus cocirculate, as shown by sequence comparisons of the HEF gene. The evolution of influenza B viruses is somewhere between these two examples, with relatively few lineages of the HA gene (but more than one) cocirculating.[59] Relatively less information is available regarding the evolution of NA gene sequences, but these appear to follow a similar pattern.[60] Antigenic Shift The major antigenic shifts that herald pandemic influenza presumably result from a different mechanism. These viruses are “new” viruses to which the population has no immunity. There is very little or no serologic relationship between the HA (or NA) antigens of the “old” and “new” viruses; hence, in nomenclature, each receives a different designation. The schema shown in Figure 162–5 ties together the concepts of antigenic shift and antigenic drift in relation to population immunity.[61] When a new virus, here called HxNx, to which antibody is lacking, is introduced into a population, pandemic influenza results. After one or more waves of pandemic influenza, the proportion of immune individuals in the population increases. This situation favors the emergence of viruses with antigenic changes in the HA and/or NA, whose spread through the partially immune population is thus facilitated. This phenomenon is repeated with subsequent epidemics due to strains of influenza A/HxNx that exhibit some antigenic drift. After 10 to 30 years of circulation of variants with this given subtype, the level of immunity in the population to all variants within the subtype is very high, and the conditions for the spread of a new virus, HyNy, become favorable, with the emergence of a new pandemic of influenza. Figure 162-5 Schema of the occurrence of influenza pandemics and epidemics in relation to the level of immunity in the population. A/HxNx and A/HyNy represent influenza viruses with completely different hemagglutinins and neuraminidases. (Modified from Kilbourne ED. The epidemiology of influenza. In: Kilbourne ED, ed. The Influenza Viruses and Influenza. New York: Academic Press; 1975:483, with permission.) The pattern of replacement of HA and NA subtypes during the most recent century of pandemics is shown in Figure 162–6 and is based both on virus isolation and serologic studies of individuals who lived through previous pandemics. Such studies suggest that the pandemic of 1889 was associated with viruses of an H2N2 type, followed by a pandemic in 1901 caused by an H3N8-type virus.[62] Virologic and polymerase chain reaction (PCR) studies have shown that the “Spanish” pandemic of 1918 (mentioned previously) was caused by an H1N1 virus, which in turn was supplanted in the “Asian” pandemic of 1957 by H2N2 viruses. In 1968, the “Hong Kong” pandemic was caused by viruses of the H3N2 subtype. In 1977, viruses of the H1N1 subtype were reintroduced through an unknown mechanism. These viruses are genetically identical to the H1N1 viruses that were circulating in 1950. Since 1977, influenza A viruses of both the H1N1 and H3N2 subtypes have cocirculated. Figure 162-6 Recent pandemics of influenza. The duration of circulation of viruses of various subtypes is shown by the boxes. Because the nature of influenza epidemics prior to 1918 is known only by serologic means, those boxes are shaded tan. Below the time line is given the ages of individuals in 2004 who were alive during the various epidemic periods of earlier influenza subtypes. For example, individuals currently living who are between the ages of 47 and 86 probably experienced their first influenza A infection as an H1N1 virus. Individuals who are 36 years of age or younger have never been infected with H2N2 viruses. The degree of genetic difference between subtypes, 30% or greater, precludes their arising by simple point mutation, and the origin of new pandemic strains has been the subject of intense interest and study, for obvious reasons. The most plausible explanation for their origin takes into account three features of this phenomenon: that the virus has a segmented genome, that pandemics occur only with influenza A viruses, and that influenza A viruses, but not other influenza viruses, maintain a large reservoir of genetic diversity in animals. Influenza A viruses infect a variety of species, including man, swine, horses, marine mammals, and in particular, birds. In fact, no less than 15 unique HA subtypes (H1 to H15)[63] and nine NA subtypes (N1 to N9)[64] have been identified in avian influenza viruses. Fortunately, avian influenza A viruses themselves appear to be relatively restricted in their ability to replicate in humans.[65] The precise molecular mechanisms responsible for the host-range preferences of avian influenza A viruses are not completely known, but several factors probably play a role. The divergent evolution of the genes of these viruses in avian hosts could have resulted in less efficient interactions between undefined viral and mammalian host cell components. The relative attenuation of avian-human influenza reassortants for man[66] supports a role for non-HA genes in this restriction. In addition, the HAs of avian and mammalian influenza viruses display a different host-cell receptor specificity, with avian viruses preferring receptors containing sialic acid–galactose linkages of the α2➙3 variety, and mammalian viruses tending toward α2➙6 linkages. Extensive sequence analysis has suggested at least two mechanisms by which avian viruses can circumvent these barriers to interspecies transmission. These studies have shown significant sequence similarity between the HA, NA, and PB1 gene segments of the pandemic H2N2 virus and avian viruses,[67][68] and between the H3 and PB1 gene segments of the pandemic H3N2 virus and avian viruses,[67][69] suggesting that in some circumstances, new pandemic viruses arise by reassortment between avian viruses, which provide novel surface glycoproteins, and human viruses, which provide genes allowing efficient replication in humans. Reassortment would be facilitated by the presence of a third species that is susceptible to infection with both avian and human viruses, such as the pig. Avianto-swine transmission has been demonstrated previously in nature,[70][71] and naturally occurring avian–human reassortant viruses have been recovered in pigs.[72] However, there are likely to be constraints on what types of reassortants are viable; in particular, it has been suggested that the hemagglutinins of recent human influenza A viruses are not compatible with the matrix genes of current avian viruses,[73] and phenomena of this type may limit the possibilities for generation of pandemic viruses by reassortment. A second mechanism would involve adaptation of avian viruses to the human host by evolution in swine, and this is supported by sequence analysis showing that the 1918 pandemic was most likely the result of direct introduction of a swine influenza A virus into humans.[74] Recently, it has been shown that avian H1N1 viruses introduced into swine populations are evolving in these animals and have switched receptor specificity to a more mammalian type.[75] This type of evolution is very likely facilitated by the presence of both types of receptors in pig tracheal epithelia.[75] Finally, avian viruses might also be directly introduced into human populations without prior reassortment or adaptation in an intermediate host. Avian viruses of three different hemagglutinin subtypes, H5, H7, and H9, have caused human disease, and in the case of the H5 and H7 viruses, some illnesses have been severe or even fatal.[76][77][78] In 1997, an outbreak of H5N1 infection occurred in Hong Kong, in which 18 people were hospitalized and six died. A second outbreak in February 2003 involved two cases and one fatality.[79] Additional human cases of H5N1 were reported in early 2004, from Vietnam and Thailand.[80] A large outbreak of H7N7 infection in The Netherlands in April of 2003 involved 87 human cases. Most of the individuals had illness restricted to conjunctivitis, but one fatality associated with severe pulmonary involvement occurred.[81] Human infection with avian H9N2 virus has also been reported.[82] Human infection appears to take place in the context of intense exposure to infected bird droppings in live bird markets or in agricultural settings. However, despite the presence of virus in the respiratory tract of infected individuals, person-to-person transmission appears to occur rarely, if ever.[83] The specific barrier to transmission is unclear, but there is considerable concern that eventually these introductions will result in generation of a transmission-competent virus. PATHOGENESIS AND HOST RESPONSE Cellular Pathogenesis Influenza virus infection is acquired by a mechanism involving the transfer of virus-containing respiratory secretions from an infected to a susceptible person.[84] A number of lines of evidence indicate that small-particle aerosols (<10 μm mass median diameter) are the predominant factor in such person-to-person transmission. First, large amounts of virus are present in respiratory secretions of infected persons at the time of illness and are thus available for dispersion in smallparticle aerosols created by sneezing, coughing, and talking.[84] Second, the explosive nature and simultaneous onset in many persons suggest that a single infected person can transmit virus to a large number of susceptible individuals. Furthermore, influenza virus type A has been shown to be relatively stable in small-particle aerosols at a variety of relative humidities and temperatures, but survival appears to be favored by low relative humidity and low environmental temperature.[85] In experimental influenza in volunteers, inoculation with smallparticle aerosols produces an illness that more closely mimics natural disease than does inoculation with large drops into the nose.[86][87] Finally, in such experimental infections, doses of 137 to 300 times the median tissue-culture infective dose (TCID50) are required to infect by nasal drops, whereas 0.6 to 3.0 TCID50 (i.e., a 100-fold lower dose) is infectious by the aerosol route.[86][87] Once virus is deposited on the respiratory tract epithelium, it can attach to and penetrate columnar epithelial cells if not prevented from doing so by specific secretory antibody (IgA), by nonspecific mucoproteins to which virus may attach, or by the mechanical action of the mucociliary apparatus. After adsorption, virus replication begins, leading to cell death through several mechanisms. There is a dramatic shutoff of host-cell protein synthesis that occurs at several levels. Newly synthesized cellular mRNAs are degraded (probably because cleavage by the virus cap endonuclease renders these transcripts susceptible to hydrolysis by cellular nuclease),[88] whereas translation of already-synthesized cytoplasmic mRNAs is blocked at both initiation and elongation.[89] Finally, expression of the influenza virus PA protein has been shown to induce generalized degradation of coexpressed proteins through an unknown mechanism.[90] Ultimately, the loss of critical cellular proteins very likely contributes to cell death. In addition to effects leading to cell necrosis, infection of cells with influenza A and B viruses causes cell death by apoptosis,[91][92] a form of cell death characterized by fragmentation of nuclear DNA. Bronchiolar epithelial and alveolar cells harvested from experimentally infected mice also exhibit apoptotic changes, suggesting that this mechanism of cell death may be important in the pathogenesis of influenza in vivo.[93] The specific mechanism by which influenza virus induces apoptosis is unclear, but it may be related to induction of Fas antigen by double-stranded RNA during virus replication.[94] An unusual viral protein of influenza A viruses, encoded by a second open reading frame in the PB1 gene and therefore referred to as PB1-F2, also plays a role in induction of apoptosis by poisoning mitochondria.[95] Virus release continues for several hours before cell death ensues. Released virus then may initiate infection in adjacent and nearby cells, so within a few replication cycles, a large number of cells in the respiratory tract are releasing virus and dying as a result of the virus replication. The time between the incubation period and the onset of illness and virus shedding varies from 18 to 72 hours depending in part on the inoculum dose.[84][96] Influenza virus infection of peripheral blood mononuclear cells, including polymorphonuclear leukocytes (PMNs), lymphocytes, and monocytes, is nonproductive, but it is associated with measurable defects in cellular function that may be relevant to the pathogenesis of influenza-related infectious complications. These include defects in PML chemotaxis and phagocytosis[97] as well as decreased proliferation and costimulation by mononuclear cells.[98][99] The effects are mediated by virus replication and possibly by a direct toxic effect of certain virus proteins, including hemagglutinin, neuraminidase,[100][101] and nucleoprotein.[102] It has been noted that the short portion of the sequence of the influenza A virus NP is homologous to a naturally occurring peptide found in normal bronchoalveolar lavage fluid that inhibits PMN chemotaxis and oxidative burst.[103] Virus Shedding Quantitation of virus in respiratory tract specimens reveals a characteristic pattern ( Fig. 162–7 ). Virus is first detected just before the onset of illness (within 24 hours), rapidly rises to a peak of 3.0 to 7.0 log10 TCID50/mL, remains elevated for 24 to 48 hours, and then rapidly decreases to low titers.[84][104] Usually, virus is no longer detectable after 5 to 10 days of virus shedding. However, because of the relative lack of immunity in the young, more prolonged shedding of higher titers of virus is seen in children.[105] Figure 162-7 Time course of virus shedding, symptoms, and cytokine responses of healthy adults after experimental inoculation with wild-type A/Texas/36/91 virus by nasal drops. A, Mean log10 virus titer (tissue-culture infective dose [TCID50]/mL nasal secretions), clinical symptom scores, and nasal mucus weights (in grams). B, Nasal cytokine levels measured by enzyme-linked immunosorbent assay (ELISA) (pg/mL lavage fluid, corrected for collection efficiency). In both graphs, multiple measurements have been combined for illustration, so that the y-axes are relative values only. Peak values reported in each assay are approximately as follows: virus titer, 3.6 log10 TCID50/mL nasal secretions; symptom score, 7.0; nasal mucus weight, 7.0 g; interleukin (IL)-6, 450 pg/mL; interferon (IFN)-α, 150 pg/mL; tumor necrosis factor (TNF)-α, 270 pg/mL; IL-8, 9000 pg/mL. (Data from Hayden FG, Fritz R, Lobo MC, et al. Local and systemic cytokine responses during experimental human influenza A virus infection: Relation to symptom formation and host defense. J Clin Invest. 1998;101:643–649.) The severity of illness correlates temporally with quantities of virus shed in experimental influenza in volunteers, thus suggesting that a major mechanism in the production of illness is cell death resulting from viral replication.[84] Although the clinical manifestations of influenza are dominated by systemic symptoms, viral replication is limited to the respiratory tract. Instead, systemic symptoms are probably due to the release of potent cytokines, such as type I interferons, tumor necrosis factor, and interleukins (ILs), by infected cells and responding lymphocytes.[104] Histopathology Bronchoscopy of individuals with typical, uncomplicated acute influenza has revealed diffuse inflammation of the larynx, trachea, and bronchi, with mucosal injection and edema.[106][107] Biopsy in these cases has revealed a range of histologic findings, from vacuolization of columnar cells with cell loss, to extensive desquamation of the ciliated columnar epithelium down to the basal layer of cells ( Fig. 162–8 ).[107][108] Individual cells show shrinkage, pyknotic nuclei, and a loss of cilia. Viral antigen can be demonstrated in epithelial cells[109] but is not seen in the basal cell layer.[110] Generally, the tissue response becomes more prominent as one moves distally in the airway.[107] Epithelial damage is accompanied by cellular infiltrates primarily composed of lymphocytes and histiocytes.[107] Histologic findings on autopsy in more severe cases show extensive necrotizing tracheobronchitis, with ulceration and sloughing of the bronchial mucosa,[108][111] extensive hemorrhage, hyaline membrane formation, and a paucity of PMN infiltration ( Fig. 162–9 ). Patients with secondary bacterial pneumonia have the changes characteristic of bacterial pneumonia in addition to the tracheobronchial findings of influenza ( Fig. 162–10 ). Recovery is associated with rapid regeneration of the epithelial cell layer and with pseudometaplasia. Figure 162-8 A small bronchus in acute influenza A infection shows ulceration and attempted regeneration of epithelium (H&E, ×100). (Courtesy of I. D. Stuard, Reading, Pa.) Figure 162-9 Lung parenchyma in primary influenza viral pneumonia shows extensive hemorrhage, acellular hyaline membrane lining alveolar ducts and alveoli, and a paucity of inflammatory cells within the alveoli (H&E, ×400). (Courtesy of I. D. Stuard, Reading, Pa.) Figure 162-10 Lung parenchyma in secondary bacterial infection (Streptococcus pneumoniae) complicating influenza A virus infection. Note the marked intra-alveolar polymorphonuclear cell exudate (H&E, ×400). (Courtesy of I. D. Stuard, Reading, Pa.) Pathophysiology Abnormalities of pulmonary function are frequently demonstrated in otherwise healthy, nonasthmatic young adults with uncomplicated (nonpneumonic) acute influenza. Demonstrated defects include diminished forced flow rates, increased total pulmonary resistance, and decreased density-dependent forced flow rates consistent with generalized increased resistance in airways less than 2 mm in diameter,[112][113] as well as increased responses to bronchoprovocation.[112] In addition, abnormalities of carbon monoxide diffusing capacity[114] and increases in the alveolar-arterial oxygen gradient[115] have been seen. Of note, pulmonary function defects can persist for weeks after clinical recovery. Influenza in asthmatics[116] or in patients with chronic obstructive disease[117] may result in acute declines in forced expiratory vital capacity (FVC) or forced expiratory volume in 1 second (FEV1). Individuals with acute influenza may be more susceptible to bronchoconstriction from air pollutants such as nitrates.[118] Primary viral pneumonia is an uncommon but frequently severe complication of acute influenza. In this situation, virus infection reaches the lung either by contiguous spread from the upper respiratory tract or by inhalation. The trachea and bronchi contain bloody fluid, and the mucosa is hyperemic.[119] Tracheitis, bronchitis, and bronchiolitis are seen, with loss of normal ciliated epithelial cells. Submucosal hyperemia, focal hemorrhage, edema, and cellular infiltrate are present. The alveolar spaces contain varying numbers of neutrophils and mononuclear cells admixed with fibrin and edema fluid. The alveolar capillaries may be markedly hyperemic with intra-alveolar hemorrhage. Acellular hyaline membranes line many of the alveolar ducts and alveoli.[119] Pathologic findings seen by biopsy of lung in nonfatal cases are similar to those described in fatal cases.[120] Bacterial superinfection is a well-recognized complication of viral pneumonia and accounts for a large proportion of the morbidity and mortality of viral lower respiratory tract disease, especially in adults. Consequently, the spectrum of disease and pathophysiology of bacterial superinfection has been studied intensively, and a number of factors have been identified in viral respiratory disease that could play a role in increasing the risk of bacterial infection.[121] Uncomplicated influenza is associated with significant abnormalities in ciliary clearance mechanisms.[122][123] In addition, increased adherence of bacteria to virusinfected epithelial cells has been demonstrated.[124][125] The disruption of the normal epithelial cell barrier to infection, and loss of mucociliary clearance undoubtedly enhance bacterial pathogenesis.[107][126] In addition, influenza infection may upregulate certain cell surface receptors involved in bacterial adherence.[127] Alterations in PMNs and mononuclear cells, described earlier, may also contribute to enhanced bacterial infection.[98][128][129] Viral Factors That Influence Pathogenicity Clinical characteristics of illness during the 1918 influenza pandemic differed from those of subsequent pandemics, with higher mortality rates in young adults. The viral factors, if any, that might have been responsible for this behavior remain unknown. Extensive analysis of genetic sequences recovered from preserved specimens of material from victims of the pandemic have not revealed obvious differences between the 1918 influenza virus and more conventional influenza viruses, but investigations are continuing. Since that time, there has been little direct evidence for major inherent differences in viral strains as regards to pathogenic potential in humans. Instead, the severity of epidemics is most likely determined largely by the status of immunity in the population. However, in certain situations, individual viral proteins have been demonstrated to have a significant impact on pathogenicity. This is particularly true for the HA and NS1 proteins. An essential feature of influenza A virus replication is that proteolytic cleavage of the HA is required to generate infectious virus, and this plays a role in the most clear-cut demonstration of the role of an individual influenza virus protein in pathogenicity. Infection of fowl with avian influenza viruses can result in a relatively avirulent, asymptomatic infection limited to the respiratory and gastrointestinal mucosa, or in a virulent, rapidly progressive, fatal systemic infection with involvement of the brain and other visceral organs. Comparison of the HAs of virulent and avirulent strains of H5 subtype and H7 subtype influenza A viruses has shown that the structure of the HA cleavage site is critical in determining the virulence phenotype in this model. Proteases capable of cleaving the HA of avirulent viruses, such as tryptase Clara,[130] are restricted in distribution to cells of the respiratory and gastrointestinal mucosa, thereby limiting replication to these areas. However, addition of several basic amino acids to the cleavage site,[131] coupled with the absence of a nearby glycosylation site,[132] renders the hemagglutinin capable of being cleaved by ubiquitous cellular furin-like proteases[133] and allows these viruses to escape the confines of the mucosa and replicate systemically in chickens.[134] As described previously, human infections with H5 and H7 viruses can be fatal, and as it turns out, these viruses also have the highly cleavable form of hemagglutinin. Although some of these viruses also have a high level of lethality in mice, to date there has been no evidence of replication of these viruses outside the respiratory tract in man. Thus, the potential role of HA cleavability in pathogenesis in humans is currently unknown. Interestingly, evaluation of the nucleotide sequence of the HA from the 1918 pandemic virus did not reveal this virus to have the highly cleavable type of HA.[74] Both influenza A and influenza B viruses use the NS1 protein as a mechanism to circumvent the host type-I interferon response. The NS gene antagonizes the action of type-I interferons through an unknown mechanism, and absence of the NS1 protein renders the virus incapable of growth in interferon-competent systems. The NS gene of the H5 avian viruses appears to be especially potent in this regard, and this may provide a partial explanation for its enhanced virulence in mice. Recent reports have suggested that H5 viruses associated with fatal cases in humans have changes in nucleotide sequences in the N51 gene that result both in increased resistance to the action of interferon and in the ability to induce proinflammatory cytokines.[135] In contrast, when the NS gene of the 1918 pandemic human virus was placed in the background of an avirulent influenza virus and administered to mice, more attenuated disease, rather than enhanced disease, was the result, suggesting the effect is host specific. Multiple additional animal models have been described in which it is possible to generate influenza viruses with altered levels of pathogenicity. A variety of classic genetic and molecular biologic techniques have been used to evaluate the role of specific viral genes or gene products in determining the virulence of influenza viruses in these models. An exhaustive review of these studies is beyond the scope of this chapter, but they have generally shown that virulence is a multigenic trait whose specific basis varies with the virus strains and the models used.[136][137][138][139] Immunology Epidemiologic and experimental observations in humans have shown that infection with influenza virus results in long-lived resistance to reinfection with the homologous virus.[140] In addition, variable degrees of cross-protection within a subtype have been observed, but infection induces essentially no protection across subtypes,[141] or between types A and B. Infection induces both systemic and local antibody, as well as cytotoxic T-cell responses, each of which plays a role in recovery from infection and resistance to reinfection. Antibody Responses Systemic Antibody Responses. Infection with influenza virus results in the development of antibody to the influenza virus envelope glycoproteins HA and NA, as well as to the structural M and NP proteins. Some individuals may develop antibody to the M2 protein as well.[142] As measured by enzyme-linked immunosorbent assay (ELISA), serum IgM, IgA, and IgG antibody to the HA appear simultaneously within 2 weeks of inoculation of virus.[143] The antibody response is more rapid after reinfection. The development of anti-NA antibodies parallels that of hemagglutinin-inhibiting (HAI) antibodies.[144] However, whereas responses to the HA develop after primary infection, responses to the NA appear to require previous infection.[141] Peak antibody responses are seen at 4 to 7 weeks after infection and decline slowly thereafter; titers can still be detected years after infection even without reexposure. Antibody to the HA can be measured by standard HAI tests or a variety of ELISAs, and it neutralizes virus infectivity.[145] Because of the cost and requirement for cell cultures for the neutralizing test, the HAI test is the primary method of detecting antigenic relatedness among hemagglutinins of influenza viruses. Antihemagglutinin antibody protects against both disease and infection with the homologous virus.[146] Although there is no exact correlation, serum HAI titers of 1:40 or greater, or serum neutralizing titers of 1:8 or greater, are associated with protection against infection; HAI titers of 1:20 or 1:10 are associated with lesser degrees of protection. Higher levels of antibody may be required for complete protection in older adults.[147][148] Protection in clinical studies has been shown to be primarily strain specific, but some degree of protection is present against strains showing antigenic drift within a subtype, depending on the degree of drift.[149][150] For example, Foy and colleagues showed that influenza A vaccine (A/Hong Kong/68/H3N2) induced protection against the drift variant A/England/72/H3N2 virus that persisted for 3 years.[149] Generally, antibody that is present in low quantity, or that is primarily directed against a heterologous strain of influenza, may only modify the severity of illness and not prevent infection. Antibody to the NA can be measured by NA inhibition or ELISA. In contrast to anti-HA antibody, anti-NA antibody does not neutralize virus infectivity but instead reduces efficient release of virus from infected cells, resulting in decreased plaque size in in vitro assays[151] and in reductions in the magnitude of virus shedding in infected animals.[152][153] Observations on the relative protection of those with anti-N2 antibody during the A/Hong Kong/68 (H3N2) pandemic,[144][154] as well as experimental challenge studies in humans,[155] have shown that anti-NA antibody can be protective against disease and results in decreased virus shedding and severity of illness, but that it is infection permissive.[156] Passive transfer studies in mice have also suggested that antibody to the M2 protein of influenza A viruses may have a similar effect to that of anti-NA antibody.[157] Antibody to internal viral proteins such as M or NP can be measured by the complement fixation (CF) test. These antibodies are cross-reactive among type A viruses, but they are non-neutralizing and do not appear to play a role in protective immunity. They disappear much more rapidly (in weeks to months) than do neutralizing, HAI, or anti-NA antibodies, primarily because they are predominantly IgM rather than IgG, and thus they may be useful for diagnosis of recent infection. Mucosal Antibody Responses. The majority of studies of mucosal responses to influenza in humans have concentrated on measurement of HA responses by ELISA or by neutralization tests, because nonspecific inhibitors of hemagglutination present in nasal mucus interfere with the standard HAI test. These studies have demonstrated significant mucosal responses to infection with wild-type virus or live-attenuated influenza vaccines. Both IgA and IgG are found in nasal secretions. Nasal HA-specific IgG is predominantly IgG1, and its levels correlate well with serum levels of HAspecific IgG1, suggesting that nasal IgG originates by passive diffusion from the systemic compartment.[158] Nasal HA-specific IgA is predominantly polymeric and IgA1, suggesting local synthesis. Serum HA-specific IgA is also mostly polymeric IgA1. The origin of serum IgA after mucosal infection is unclear but may derive from seeding of peripheral lymphoid tissue by memory cells derived from the mucosa.[146] Studies in mice and ferrets have emphasized the importance of local IgA antibody in resistance to infection, particularly in protection of the upper respiratory tract. Polymeric IgA was shown to be specifically transported into the nasal secretions of mice and to protect against nasal challenge. Protection could be abrogated by intranasal administration of antiserum against IgA but not IgM or IgG.[159] Local antibody has also been shown to play a role in protection against antigenic variants in mice.[160] Studies in humans have also suggested that the resistance to reinfection induced by virus infection is mediated predominantly by local HAspecific IgA, whereas that induced by parenteral immunization with inactivated virus depends also on systemic IgG.[155][161] Almost all persons with nasal neutralizing antibody titers of 1:4 or greater are protected against influenza.[162][163] Importantly, either mucosal or systemic antibody alone can be protective if present in high enough concentrations, and optimal protection occurs when both serum and nasal antibodies are present.[164] Cellular Responses Antibody responses to the HA are T-cell dependent[165][166][167] and class II restricted. CD4+ cells provide help (Th) to B cells for production of antibody to the HA and NA. Both CD4+ cells that recognize epitopes on the HA molecule, and CD4+ cells that recognize epitopes on M, NP, or PB2 may provide help for HA antibody production.[168] The epitopes on HA recognized by Th cells are distinct from those recognized by neutralizing antibody,[169] and they may be cross-reactive within a subtype. Influenza-specific Th cells also promote the generation of virusspecific CD8+ cytotoxic T lymphocytes.[170][171] Recently, it has been recognized that Th responses can be further classified as type 1 (Th1) or type 2 (Th2) responses on the basis of the profile of cytokines produced on in vitro challenge. Influenza virus infection of mice generates a strong Th1-type response.[172] Th2-type cytokines (IL-4, IL-5, IL-6, IL-10) have been also described in the lungs of mice infected with influenza virus.[173][174] Circumstantial evidence suggests that protective immune responses to influenza are associated with Th1like responses. Adoptive transfer of anti-influenza T-cell clones secreting cytokines of the Th2 type fails to promote viral clearance,[175] and administration of interferon-γ delays viral clearance and development of CTL in influenza virus– infected mice.[176] Of note, blockade of gamma interferon by interferon antibody does not affect development of CTL responses but results in reduced migration of PMNs to the lung in the murine model.[177] In addition, administration of IL-4 to infected mice promotes Th2-type responses and results in markedly delayed viral clearance.[178] Influenza virus–infected cells can be lysed by antibody in the presence of complement, by antibody-dependent cellular cytotoxicity,[179] or by the action of cytotoxic T (Tc) lymphocytes. Generally, Tc lymphocytes express CD8+ and recognize class I HLA. Such cells may recognize either HA or internal proteins such as M, NP, or PB2.[180] Therefore, Tc lymphocytes may be subtype specific or, in the case of those that recognize internal proteins, may be broadly cross-reactive, lysing cells infected with influenza A but not influenza B virus.[181][182] In addition, class II restricted cells may exhibit cytotoxic activity similar to that shown by class I restricted cells.[182] Extensive adoptive transfer experiments have shown that virus-specific Tc lymphocytes can mediate recovery from influenza virus [183][184][185][186][187][188] infection, including both HA-specific and cross-reactive Tc. However, studies in mice lacking major histocompatibility complex (MHC) class I have shown that Tc lymphocytes are not absolutely required for recovery.[189][190][191] Tc lymphocyte responses to influenza also develop in humans after influenza virus infections, generally peaking on about day 14 after infection.[192] Although not studied extensively, the presence of virusspecific prechallenge, class I–restricted Tc lymphocytes has been shown to correlate with reductions in the duration and level of virus replication in adults with low levels of serum HA and NA antibody who were challenged with influenza A virus.[193] The role of Tc lymphocytes directed against internal viral proteins in protection against severe disease in humans is unclear, as the internal virus proteins were shared between viruses causing the pandemics of 1957 and 1968 and the viruses in circulation immediately prior to these pandemics.[68][194] Memory Tc-lymphocyte responses may play a role in ameliorating the severity of disease and speeding recovery after infection, as suggested by the finding of more severe influenza in individuals with severe defects in cell-mediated immunity.[28] CLINICAL FINDINGS Uncomplicated Influenza Typical uncomplicated influenza often begins with an abrupt onset of symptoms after an incubation period of 1 to 2 days. Many patients can pinpoint the hour of onset.[84][195][196][197] Initially, systemic symptoms predominate, including feverishness, chilliness or frank shaking chills, headaches, myalgia, malaise, and anorexia. In more severe cases, prostration is observed. Usually, myalgia or headache is the most troublesome symptom, and the severity is related to the height of the fever. Myalgia may involve the extremities or the long muscles of the back. In children, calf muscle myalgia may be particularly prominent. Severe pain in the eye muscles can be elicited by gazing laterally, and arthralgia but not frank arthritis is commonly observed. Other ocular symptoms include tearing and burning. The systemic symptoms usually persist for 3 days, the typical duration of fever. Respiratory symptoms, particularly a dry cough, severe pharyngeal pain, and nasal obstruction and discharge, are usually also present at the onset of illness but are overshadowed by the systemic symptoms. The predominance of systemic symptoms is a major feature distinguishing influenza from other viral upper respiratory infections. Hoarseness and a dry or sore throat may also be present, but these symptoms tend to appear as systemic symptoms diminish, and thus they become more prominent as the disease progresses, persisting 3 to 4 days after the fever subsides. Cough is the most frequent and troublesome of these symptoms and may be accompanied by substernal discomfort or burning. Older adults may simply present with high fever, lassitude, and confusion without the characteristic respiratory complaints, which may not occur at all. In addition, there is a wide range of symptomatology in healthy adults, ranging from classic influenza to mild illness or asymptomatic infection. Fever is the most important physical finding. The temperature usually rises rapidly to a peak of 100° to 104° F, and occasionally to 106° F, within 12 hours of onset, concurrent with the development of systemic symptoms. Fever is usually continuous but may be intermittent, especially if antipyretics are administered. On the second and third days of illness, the temperature elevation is usually 0.5° to 1.0° F lower than on the first day, and as the fever subsides, the systemic symptoms diminish. Typically, the duration of fever is 3 days, but it may last 4 to 8 days. In a small number of cases, a second fever spike occurs on the third or fourth day and results in a biphasic fever curve. Early in the course of illness, the patient appears toxic, the face is flushed, and the skin is hot and moist. The eyes are watery and reddened. A clear nasal discharge is common, but nasal obstruction is uncommon. The mucous membranes of the nose and throat are hyperemic, but exudate is not observed. Small, tender cervical lymph nodes are often present. Transient scattered rhonchi or localized areas of rales are found in less than 20% of cases. A convalescent period of 1, 2, or more weeks to full recovery then ensues. Cough, lassitude, and malaise are the most frequent symptoms during this period. Available data suggest that illness associated with influenza B virus infection closely resembles that described for influenza A, although some have suggested that influenza B illness may be somewhat milder than influenza A illness.[198][199] In contrast, influenza C infection, when it occurs, causes afebrile common colds and rarely, if ever, produces the influenza syndrome.[200] It does not occur in epidemics. At the extremes of age, there are prominent differences in influenza. Influenza attack rates are higher in children than in adults.[23][201] Maximal temperatures tend to be higher among children, and cervical adenopathy is more frequent among children than among adults.[96] Croup associated with influenza virus infection occurs only among children.[202][203][204] Among older adults, fever remains a very frequent finding, although the height of the febrile response may be lower than among children and young adults. Pulmonary complications are far more frequent in older adults than in any other age group. Complications of Influenza Pulmonary Complications Two manifestations of pneumonia associated with influenza are well recognized: primary influenza viral pneumonia and secondary bacterial infection. In addition, less distinct and milder pulmonic syndromes often occur during an outbreak of influenza that may represent tracheobronchitis, localized viral pneumonia, or possibly mixed viral and bacterial pneumonia. Comparative features of these clinical syndromes are shown in Table 162–4 . Studies to determine the interaction between virus and bacteria have helped researchers understand the different clinical patterns described here.[205] Table 162-4 -- Comparative Features of Pulmonary Complications of Influenza Secondary Mixed Viral and Localized Primary Viral Bacterial Bacterial Viral Pneumonia Pneumonia Pneumonia Pneumonia Setting Cardiovascular disease; Age >65 yr; Any associated pregnancy; ?Normal pulmonary disease with A or B young adult (Hsw1N1) Clinical history Relentless progression from classic 3-day influenza Improvement, then worsening after 3-day influenza Features of both Continuation primary and of classic 3secondary day syndrome pneumonia Bilateral Physical findings, no Consolidation examination consolidation Consolidation Area of rales Sputum Pneumococcus, Normal flora Normal flora Pneumococcus, Secondary Primary Viral Bacterial Pneumonia Pneumonia bacteriology Chest radiography Staphylococcus, Haemophilus influenzae Bilateral findings Consolidation Mixed Viral and Localized Bacterial Viral Pneumonia Pneumonia Staphylococcus, H. influenzae Consolidation Segmental infiltrate Leukocytosis White blood Leukocytosis with Leukocytosis with Usually with a shift to the cell count a shift to the left a shift to the left normal left Isolation of influenza Yes virus No Yes Yes Response to No antibiotics Yes Often No Mortality Low Variable Very low High Primary Influenza Viral Pneumonia. The syndrome of primary influenza viral pneumonia was first well documented in the 1957–1958 outbreak.[106][119] However, it is clear that many deaths of young healthy adults in the 1918–1919 outbreak were the result of this syndrome. In outbreaks since 1918, primary influenza viral pneumonia has occurred predominantly among persons with cardiovascular disease, especially rheumatic heart disease with mitral stenosis, and to a lesser extent in others with chronic cardiovascular and pulmonary disorders. The illness begins with a typical onset of influenza, followed by a rapid progression of fever, cough, dyspnea, and cyanosis. Physical examination and chest radiographs reveal bilateral findings consistent with the adult respiratory disease syndrome but no consolidation. Blood gas studies show marked hypoxia, Gram stain of the sputum fails to reveal significant bacteria, and bacterial culture yields sparse growth of normal flora, whereas viral cultures yield high titers of influenza A virus. Such patients do not respond to antibiotics and mortality is high. At autopsy, findings consist of tracheitis, bronchitis, diffuse hemorrhagic pneumonia, hyaline membranes lining alveolar ducts and alveoli, and a paucity of inflammatory cells within the alveoli (see Figs. 162–9 and 162–10 ). At the present time, late in the interpandemic era, severe primary influenza viral pneumonia is rare. Secondary Bacterial Pneumonia. Secondary bacterial pneumonia often produces a syndrome that is clinically indistinguishable from that occurring in the absence of influenza.[206][207] The patients (most often older adults or those with chronic pulmonary, cardiac, and metabolic or other disease) have a classic influenza illness followed by a period of improvement lasting usually 4 to 14 days. Recrudescence of fever is associated with symptoms and signs of bacterial pneumonia such as cough, sputum production, and an area of consolidation detected on physical examination and a chest radiograph. Gram staining and culture of sputum reveal a predominance of a bacterial pathogen, most often Streptococcus pneumoniae or Haemophilus influenzae, and, notably, an increased frequency of Staphylococcus aureus, which is otherwise an uncommon cause of community-acquired pneumonia. Such patients usually respond to specific antibiotic therapy. Analysis of different radiographic patterns of pneumonia indicates that a variety of abnormalities can occur in all ages.[208][209] During an outbreak of influenza, many patients do not clearly fit into either of the aforementioned categories.[210] The disease is not relentlessly progressive, and yet the fever pattern may not be biphasic. These patients may have primary viral, secondary bacterial, or mixed viral and bacterial infection of the lung. In more recent epidemics, in which surveillance cultures of hospitalized patients have been carried out, most patients with pneumonia and influenza presented early, while they were still culture positive for influenza virus. Most responded to antibiotics without the use of antivirals. In addition, milder forms of influenza viral pneumonia involving only one lobe or segment have been described that do not invariably lead to death, and that are more likely to be confused with pneumonia caused by Mycoplasma pneumoniae than to pneumonia produced by bacterial infection. In children, pneumonia may occur, but it is less common than in adults. Bronchitis may also occur as a result of influenza A or B virus infection, but respiratory syncytial virus and parainfluenza virus type 3 are more important causes of bronchiolitis. Pulmonary Complications in Immunosuppressed Patients. In patients with HIV infection, influenza has not been recognized as a major clinical problem, although disease of greater severity has been noted in some patients[211] and pneumonic complications of influenza have occurred. Additional studies are required to better define the importance of influenza virus infection in HIV-infected patients. Influenza has been noted to cause severe disease with an increased incidence of pneumonia in immunosuppressed children with cancer compared with agematched individuals without immunosuppression.[212] Severe disease associated with pneumonia and death has been reported, particularly in bone marrow transplant recipients and leukemic patients.[28][213][214] Relatively more immunosuppressed individuals early after transplantation appear to be at greater risk.[214] However, for reasons that are not completely clear, influenza has not appeared to be quite the problem in this population that other respiratory viruses, particularly paramyxoviruses, are. Influenza virus shedding can be quite prolonged in immunosuppressed children,[215] particularly those with HIV and low CD4+ counts.[216] Because of the prolonged, unchecked replication of influenza viruses in these individuals, resistance to antiviral drugs eventually occurs in many treated patients.[215][217] Other Pulmonary Complications. In addition to pneumonia, other pulmonary complications of influenza have been recognized. Croup. Significant numbers of cases of croup occur in influenza A and B outbreaks.[202][203] Croup associated with influenza A virus appears to be more severe but less frequent than that associated with parainfluenza virus types 1 or 3 or respiratory syncytial virus infections (see Chapters 153 and 155 ). Exacerbation of Chronic Pulmonary Disease. Acute exacerbation of chronic bronchitis, a phenomenon that is associated with other respiratory disease–causing viruses and bacteria,[218][219][220] is common. Studies by Monto and Ross have shown that such infections result in a permanent loss of pulmonary function.[221] Another major illness that is exacerbated is asthma. Often, stable asthmatics will worsen to status asthmaticus as a result of influenza.[222][223] Another illness exacerbated by influenza is cystic fibrosis. In children afflicted with this entity, influenza infections may lead to severe complications.[224] Frequency of Pulmonary Involvement. The findings of persistent physiologic changes in the lower respiratory tract with uncomplicated influenza discussed earlier suggest that viral invasion of the lower respiratory tract is common in uncomplicated influenza and may help to explain the relatively long convalescence. The frequency of overt involvement of the respiratory tract has been answered in part by Fry.[208][209][225] In five successive epidemics, he showed that the overall rate of chest complications (tracheobronchitis or pneumonia) was 9.5% of cases. From the ages of 5 to 50, the rate was low (4% to 8%), but it increased progressively after the age of 60, reaching a level of 73% in those over 70 years of age. Foy et al. studied seven successive epidemics of influenza A infection and showed that six of the seven were associated with at least a doubling of pneumonia rates among adults.[226] Still others have studied rates of admission to the hospital and have shown a lower impact on hospitalization.[226][227][228] Nonpulmonary Complications Most of the complications of influenza have been evaluated in years when there were sizable outbreaks.[119][229][230][231] However, as antigenic variation of a subtype evolves and as the exposure of the population to vaccine and to virus occurs, the full-blown influenza syndrome becomes a less frequent manifestation. Nonetheless, infection rates may remain high, and consequences of infection in severely compromised older adult patients remain significant. Myositis. Myositis and myoglobinuria with tender leg muscles and elevated serum creatine phosphokinase (CPK) levels have been reported, mostly in children after influenza A or B infection, most commonly after the latter,[232][233][234][235] but they can occur in adults as well. Symptoms may be sufficiently severe to interfere with walking, but neurologic changes are not evident. Cardiac Complications. Both myocarditis and pericarditis have been rarely associated with influenza A or B virus infection.[236] Some investigators have associated influenza with myocardial infarction. However, neither myocarditis nor pericarditis is commonly observed at autopsy among those who died of primary influenza viral pneumonia.[119] In patients with cardiac disease, the acquisition of influenza provides a significant risk of death.[230][237][238] Toxic Shock Syndrome. In recent outbreaks of influenza A or B, a toxic shock–like syndrome has occurred in previously healthy children or adults, presumably because viral infection changed colonization and replication characteristics of the toxin-producing staphylococcus.[239][240] Central Nervous Complications. Guillain-Barré syndrome has been reported to occur after influenza A infection, as it has after numerous other infections, but no definite etiologic relationship has been established. In addition, cases of transverse myelitis and encephalitis have occurred rarely.[241][242] An etiologic association of these syndromes with influenza virus infection has only infrequently been proven, and influenza infection accounts at most for only a small proportion of cases of each of these symptoms. Early reports of deaths associated with influenza A infection in children and young adults during the 2003–2004 season, have implicated encephalitis as a prominent feature.[243] Reye’s Syndrome. Reye’s syndrome is associated with many viral infections, prominently including influenza and varicella in children. The classic manifestation is a change in mental status occurring several days after a typical respiratory illness. Manifestations range from lethargy to delirium, obtundation, seizures, and respiratory arrest. Lumbar puncture reveals normal protein values and normal cell counts, confirming the presence of encephalopathy rather than encephalitis or meningoencephalitis. The most frequent laboratory abnormality is elevation of the blood ammonia value, which occurs in almost all patients. Reye’s syndrome is almost exclusively seen in children who have been given aspirin to treat febrile illnesses due to influenza and other viruses, and it is important to use other antipyretics such as nonsteroidal antiinflammatory drugs in this situation. Children who require continuous aspirin therapy are an important target group for influenza vaccination to reduce the risks of Reye’s syndrome. DIAGNOSIS Virus Isolation Virus isolation or detection of viral antigen in respiratory secretions is the technique of greatest utility in the setting of acute illness. Virus can be isolated readily from nasal swab specimens, throat swab specimens, nasal washes, or combined nose and throat swab specimens. The general consensus is that throat swab alone is probably less sensitive for detection than other samples. Virus can also be isolated from sputum samples, if these are being produced.[244] Samples should be placed into containers of viral transport medium and transported to the laboratory as soon as possible, although the virus survives overnight if the specimen is kept on ice. Specimens for influenza are inoculated onto rhesus monkey kidney, cynomolgus monkey kidney, or Madin-Darby canine kidney cell cultures, where virus is detected by cytopathic effect or hemadsorption. Less commonly, embryonated eggs can be used for virus isolation. Over 90% of positive cultures can be detected within 3 days of inoculation[245] and the remainder by 5 to 7 days. Rapid Diagnosis A variety of techniques have been employed to speed the process. The most widely used tests are based on immunologic detection of viral antigen in respiratory secretions. For influenza, such tests include the Directigen Flu A+B (BectonDickenson), Flu OIA (BioStar), and QuickVue Influenza A+B test (Quidel Corporation). In each of these tests, a sample of respiratory secretions is treated with a mucolytic agent and then tested, either on a filter paper (Directigen), in an optical device (Flu OIA), or with a dipstick (QuickVue) in which reaction with specific antibody results in a color change. In a slight variation of this strategy, the ZstatFlu (ZymeTx) test detects the presence of viral neuraminidase activity in the sample using a chromogenic substrate; this test is based on the same chemistry used to develop neuraminidase inhibitors. All of the tests are designed to detect both influenza A and influenza B, are relatively simple to perform, and can provide results within 30 minutes. Currently, both the QuickVue and Zstat tests are eligible for Clinical Laboratory Improvement Amendment of 1998 (CLIA) waiver. Additional tests are in development, and updated information is available at http://www.cdc.gov/flu/professionals/labdiagnosis.htm . The reported sensitivities of each test in comparison to cell culture have ranged between 40% and 80%, and they are somewhat dependent on the nature of the samples tested and the patients from whom they were derived.[246][247][248][249][250][251] In general, sensitivities in adults and older adult patients tend to be lower than those reported in young children, who shed much larger quantities of virus in nasal secretions and therefore have much higher concentrations of antigen in their samples.[252] Similarly, sensitivity is likely to be higher early in the course of illness, when viral shedding is maximal. The sensitivity of some tests for detection of influenza B viruses may be lower than that for influenza A viruses.[247][251] Reported specificities have ranged from 85% to 100%. It is important to note in this regard that in each of these studies, a portion of samples that are negative by culture and positive by rapid test are confirmed as positive by PCR (see later).[245] Although all types of respiratory samples can be used in such tests, the sensitivity appears to be better with nasopharyngeal swabs and aspirates than with throat swabs or gargles.[246][253] As no published comparative data are currently available that conclusively demonstrate superiority of one test over another, decisions regarding a specific test are generally made on the basis of convenience, cost, and the familiarity of the operator with the technique. In most medical centers, the cost is approximately $20 per diagnostic test. A variety of approaches to direct detection of viral nucleic acids in clinical specimens have also been explored for rapid diagnosis, including nucleic acid hybridization and PCR amplification. PCR in particular has the advantage of being potentially more sensitive than cell culture, and it may allow detection of virus in samples in which the virions have lost viability. In addition, it is possible to devise multiplex techniques so that a single test can detect a number of different agents.[254] However, as PCR techniques are more labor intensive and technically demanding, and they require specialized laboratory equipment, they generally have not supplanted antigen detection for rapid diagnosis. Serology Serologic tests, such as complement fixation and hemagglutination inhibition, can be used to retrospectively establish a diagnosis of influenza infection. Because most individuals have been previously infected with influenza viruses, a single serum is generally not adequate, and paired serum specimens, consisting of an acute and a convalescent sera obtained 10 to 20 days later, should be submitted for testing. Epidemiologic Diagnosis A diagnosis can also be made on epidemiologic grounds. That is, when the presence of influenza virus is confirmed in a region or community, healthy adults with acute influenza-like illness most commonly have influenza. In fact, several studies have shown that the accuracy of a clinical diagnosis in healthy adults in the setting of an influenza outbreak is as high as 80% to 90%.[255][256][257] In an analysis of symptoms in young adults being assessed for entry into studies of influenza virus treatment, the best multivariate predictors of laboratory-confirmed influenza virus infection were cough and fever,[256] with an increasing predictive value with increasing levels of fever. However, the predictive value of such a symptom complex may be less in older adults[258] and in children.[259] In nursing homes, the presence of cocirculating pathogens (such as respiratory syncytial virus) that can result in identical symptoms can clearly complicate the ability to make a clinical diagnosis of influenza specifically.[260][261] Role of Rapid Diagnosis in Clinical Decision Making The optimal use of rapid diagnostic tests in patient management is yet to be defined. Such tests are most clearly useful in the rapid identification of outbreaks within institutions or in the community, where the testing of multiple specimens can compensate for the relative lack of sensitivity of the test for any single specimen. The utility of rapid testing in other situations depends on a number of factors beyond the specific performance of the test, including the extent of influenza epidemic activity (i.e., the a priori likelihood of infection) and the potential consequences of a positive or negative result. TREATMENT Uncomplicated Influenza Four antiviral drugs are currently available for the prevention and treatment of influenza. A comparison of the basic pharmacology and antiviral activity of these agents is given in Table 162–5 , and they are described in detail later. Certain general principles apply regardless of the specific form of therapy chosen. It is important to recognize that individuals with an intact immune system who have had previous influenza infections rapidly limit the replication of these viruses. Therefore, the opportunity to impact viral replication with antiviral agents is limited, and effective use of these agents requires early initiation of therapy. No studies have ever demonstrated a benefit of antiviral therapy begun after 48 hours or more of symptoms, and the greatest effect is typically seen when therapy is started in the first 24 hours. The question of whether delayed therapy may be useful in selected populations, such as immunosuppressed individuals, remains unanswered. Table 162-5 -- Antiviral Agents for Influenza Amantadine Rimantadine Zanamivir Oseltamivir Protein target M2 M2 Neuraminidase Neuraminidase Activity A only A only A and B A and B Side effects CNS (13%) GI (6%) ?Bronchospasm GI (9%) GI (3%) GI (3%) Metabolism None Multiple (hepatic) None Hepatic Excretion Renal Renal others Renal Renal (tubular secretion) Drug interactions Antihistamines, None anticholinergics None Probenecid (increased levels of oseltamivir) None CrCl < mL/min Dose adjustments ≥65 yr old needed CrCl < mL/min Contraindications Acute-angle glaucoma + ≥65 yr old 50 CrCl < 10 mL/min 30 Severe liver dysfunction Severe liver Underlying dysfunction airways disease FDA Approved Indications Therapy Prophylaxis Adults and children ≥ year Adults only of age Yes Yes Adults and Adults and children ≥ 7 children ≥ 1 years of age year of age No Adults and children ≥ 13 years of age CNS, central nervous system; CrCl, creatinine clearance; FDA, U.S. Food and Drug Administration; GI, gastrointestinal. M2 Inhibitors: Amantadine and Rimantadine Mechanism of Action and Activity The M2 inhibitors amantadine and rimantadine are related primary symmetrical amines and are active against all strains of influenza A virus in a variety of cell culture systems and animal models.[262] In cell culture, inhibitory levels for influenza A virus range from 0.2 to 0.4 μg/mL for amantadine, and from 0.1 to 0.4 μg/mL for rimantadine.[263] The antiviral activity of these drugs is the result of inhibition of the M2 ion channel activity of susceptible viruses. The function of the M2 ion channel in viral replication is to acidify the interior of the virion, disrupting the interaction between the matrix and nucleoproteins, and allowing the ribonucleoproteins to be transported to the nucleus, where replication occurs.[264] Thus, the antiviral effect is primarily manifested in cell culture as inhibition of virus uncoating.[265][266] Similar ion channels have been described for influenza B and C viruses; however, at clinically achievable levels, these drugs are active against only influenza A. Pharmacology and Side Effects Although the mechanism of action and spectrum of activity for amantadine are similar to those for rimantadine, there are important pharmacokinetic differences between the two drugs.[267] Amantadine does not undergo metabolic change and is excreted unchanged in the urine with a half-life of 12 to 18 hours. This leads to rapid accumulation of amantadine in two settings: in patients with renal failure and in older adults with reduced renal function because of age. In older adults, it is recommended that the dosage of amantadine be reduced to no more than 100 mg daily and perhaps even to 100 mg every other day after the first few days, although extensive evidence of the efficacy for these lower doses is not available. By contrast, rimantadine undergoes extensive metabolism. Less than 15% of the drug is excreted in the urine unchanged, and the remainder is excreted as metabolic products.[268] A dosage reduction to a maximum of 100 mg/day in older adults is also recommended for rimantadine. The most common side effects of amantadine are minor and reversible central nervous system (CNS) side effects such as insomnia, dizziness, and difficulty in concentrating.[269][270][271] These side effects may be more troublesome in older adults, in whom confusion is noted in about 18% of recipients.[272] In addition, amantadine use has been associated with seizures in individuals with prior seizure disorder.[273] Minor gastrointestinal complaints have also been reported. The CNS effects of amantadine are increased when these drugs are co-administered with anticholinergics or antihistamines. In addition, trimethoprim-sulfamethoxazole may inhibit tubular secretion of amantadine and increase the potential for CNS toxicity.[274] There are no other known significant drug interactions with amantadine. However, co-administration of amantadine with drugs known to have CNS side effects may exacerbate those effects and thus should be avoided. Rimantadine is associated with a considerably reduced rate of CNS side effects, and in comparative studies of long-term administration, the rate of CNS side effects was not significantly different from the rate with placebo.[271] There are no known drug interactions that significantly affect the levels or metabolism of rimantadine. Efficacy Both amantadine and rimantadine are effective in the therapy of experimentally induced and naturally occurring influenza A. Amantadine treatment of H3N2 influenza A during the 1968 pandemic within the first 48 hours of illness was associated with decreases in the duration of fever by about 24 hours[275] and with a greater proportion of subjects considered to be “rapid resolvers.”[276][277] In addition, treated individuals had more rapid decreases in individual symptoms of cough, sore throat, and nasal obstruction.[278] Treatment with amantadine results in significantly more rapid improvement in small airways dysfunction in healthy adults with uncomplicated H3N2 influenza.[112][270] Additional trials of amantadine therapy were performed when H1N1 viruses reappeared in the late 1970s, with similar results. Early amantadine therapy of influenza A/USSR/77 in otherwise healthy adults was shown to result in a more rapid decrease in fever, and in a higher frequency of subjects reporting improved symptoms at 48 hours compared with placebo.[269] In addition, treated subjects were less likely to shed virus at 48 hours. In a second study conducted in young adults infected with A/Brazil/78, amantadine therapy was associated with a more rapid decrease in symptoms compared with aspirin therapy,[279] and with decreased virus shedding. Studies of rimantadine therapy of acute influenza in otherwise healthy adults with uncomplicated influenza have shown levels of benefit essentially identical to those seen with amantadine. Treatment of adults with H1N1[269] and H3N2[280] influenza A resulted in improved symptoms, decreased fever, and reduced virus shedding compared with placebo. When rimantadine and amantadine were directly compared in a randomized trial,[269] the efficacies of the two drugs were essentially identical. Neither amantadine nor rimantadine has been subjected to extensive efficacy evaluation in high-risk subjects. One placebo-controlled study carried out with nursing home residents showed more rapid reduction in fever and in symptoms in rimantadine recipients. Furthermore, physicians who were caring for these patients, but who were blinded to study drug status, prescribed significantly fewer antipyretics, antitussives, and antibiotics and obtained fewer chest radiographs for the rimantadine recipients.[281] Rimantadine has also been evaluated in the treatment of influenza A in children, and shown to reduce the level of virus shedding early in infection when compared with acetaminophen.[282][283] More variable effects on clinical symptom scores have been seen, with one study showing a decrease in scores and fever compared with acetaminophen,[282] and the other, in which illness was relatively mild, showing no significant difference.[283] However, rimantadine is not currently licensed for treatment of children in the United States. Drug Resistance Drug resistance has been a factor in limiting the more widespread use of these antiviral agents.[284] Although resistant viruses are seen in less than 1% of unexposed individuals,[285][286] they emerge fairly frequently in treated individuals,[287][288] particularly children.[282] Resistance is the result of single point mutations in the membrane-spanning region of the M2 protein, and it confers complete cross-resistance between amantadine and rimantadine.[289] Resistant virus can be transmitted to, and can cause disease in, susceptible contacts.[287][288][290] Prolonged shedding of resistant viruses may occur in immunocompromised patients, particularly children, and may continue even after therapy is terminated,[291] consistent with the relative fitness of these resistant viruses. Although vaccination combined with amantadine treatment can decrease the generation and transmission of resistant viruses,[292][293] the problem of drug resistance remains an important consideration and has limited enthusiasm for more widespread use of these agents. Neuraminidase Inhibitors: Zanamivir and Oseltamivir Knowledge of the crystal structure of neuraminidase complexed with its substrate, sialic acid,[294] has allowed the development of a series of sialic acid analogues with neuraminidase-inhibiting activity.[295] Mechanism of Action and Activity The neuraminidase inhibitors act by inhibiting the functioning of the influenza virus neuraminidase. This enzyme cleaves terminal sialic acid from sialic acid– containing glycoproteins that serve as host cell receptors for attachment of influenza viruses. As virus replication proceeds within the cell, neuraminidase is synthesized and transported to the cell surface, where it removes the sialic acid from these cell surface glycoproteins. Destruction of these receptors by neuraminidase is critical in allowing newly formed viruses to subsequently egress from the cell and spread to other cells. Studies with mutant, neuraminidasedeficient viruses have shown that in the absence of a functional neuraminidase, virus remains attached to the host cell and to other virions.[296][297] In addition, neuraminidase may be important in facilitating the penetration of virus through secretions in the respiratory tract, which are rich in sialic acid–containing macromolecules.[64] Neuraminidase inhibitors are active against influenza viruses at millimolar concentrations or less. Activity against clinical isolates assessed in plaque inhibition tests ranges from concentrations of 0.01 to 16 μM. Influenza B viruses are approximately 10-fold less sensitive than influenza A viruses, but they are still sensitive well within clinically achievable concentrations. Among the influenza viruses sensitive to neuraminidase inhibitors are avian viruses with all nine known neuraminidase subtypes. Pharmacology and Side Effects Although zanamivir and oseltamivir have identical mechanisms of action and similar profiles of antiviral activity, they have different pharmacologic properties. Zanamivir (4-guanidino-Neu5Ac2en) is a polar molecule that is not orally bioavailable. Therefore, effective use of this agent requires local administration. The drug is currently supplied as a dry powder for oral inhalation, using the Diskhaler device (GlaxoSmithKline) also used commonly for a variety of asthmarelated medications. Oseltamivir carboxylate is an orally bioavailable ethyl ester prodrug of oseltamivir phosphate, a carbocyclic transition-state–based inhibitor of the influenza virus neuraminidase.[298] Oseltamivir is rapidly absorbed from the gastrointestinal tract and is converted in the liver by hepatic esterases to the active metabolite, oseltamivir carboxylate. The metabolite is excreted unchanged in the urine by tubular secretion, with a serum half life of 6 to 10 hours. Administration of the drug with food may improve tolerability without impacting drug levels. Zanamivir is not bioavailable by the oral route and must be administered topically to be effective. The drug is supplied in blister packs in which each blister contains 5 mg of zanamivir and 20 mg of lactose carrier. The standard dose is therefore two inhalations twice a day. It is estimated that approximately 4 mg of drug is actually delivered with each inhalation. Intravenous dosing of zanamivir has also been studied, although this formulation is not currently available for clinical use. In one small study, an intravenous dose of 600 mg twice daily was well tolerated and was effective in preventing experimental infection of adults with influenza A (H1N1) virus.[299] Both drugs have been well tolerated in clinical trials. The major adverse effects reported for oseltamivir have been gastrointestinal upset, probably irritation due to rapid release of the drug in the stomach. Rates of nausea can be substantially reduced if the drug is taken with food. The most commonly reported adverse effects in individuals treated with zanamivir have been diarrhea, nausea, and nasal signs and symptoms, which have occurred at essentially the same rate as in placebo recipients. In one study in which zanamivir was used in influenza-infected patients with asthma or chronic obstructive pulmonary disease, the frequency of significant changes in FEV1 or peak flow rates was higher in zanamivir than in placebo recipients. For this reason, individuals with these pulmonary conditions should have ready access to a rapidly acting bronchodilator when using zanamivir, in the event that the drug precipitates bronchospasm. The dose of oseltamivir should be reduced to 75 mg once daily in individuals with renal impairment (i.e., with creatinine clearance of less than 30 mL/min). No data are available regarding the use of the drug in individuals with more significant levels of renal impairment. Likewise, no information is available regarding the use of oseltamivir in individuals with hepatic impairment. Clinically significant drug interactions have not been reported. Because oseltamivir is eliminated by tubular secretion, probenecid increases serum levels of the active metabolite approximately twofold. However, dosage adjustments are not necessary in individuals taking probenecid. Co-administration of cimetidine, amoxicillin, or acetaminophen has no effect on serum levels of oseltamivir or oseltamivir carboxylate.[300] Although significant increases in the serum half-life of zanamivir are seen in the presence of renal failure, the small amounts of the drug that are absorbed systemically suggest that dosage adjustments would not be necessary. Studies of the pharmacokinetics of the drug in the presence of impaired hepatic function have not been reported. Efficacy Zanamivir and oseltamivir, the two available neuraminidase inhibitors, have shown very similar results in clinical trials. Both drugs were initially evaluated in the human experimental challenge model. Studies in which oseltamivir was administered 28 hours after experimental infection showed reductions in viral shedding, reduced symptom scores, and decreased frequencies of middle ear abnormalities compared with placebo.[301] Zanamivir given by drops or spray as late as 50 hours after infection also demonstrated reduced viral shedding, symptom scores, nasal mucus weights, and middle ear abnormalities.[302][303] Similar effects were seen with oseltamivir in adults experimentally infected with influenza B virus.[304] In studies of naturally occurring, uncomplicated influenza in healthy adults, therapy with oseltamivir initiated within the first 36 hours of symptoms resulted in 30% to 40% reductions in the duration of symptoms and severity of illness and reduced rates of prolonged coughing.[305][306] In addition, early therapy is associated with a significantly earlier return to work or other normal activities. Similarly, in healthy adults, early therapy of uncomplicated influenza A or B with inhaled zanamivir has been shown to result in a reduction of approximately 0.8 to 1.5 days in the duration of influenza symptoms, and an earlier return to normal activities.[307][308] Early treatment of healthy adults with zanamivir may also reduce the frequency of complications, with reductions in the use of antibacterials and in hospitalization.[309] Both oseltamivir and zanamivir have been evaluated as therapy for children, but only oseltamivir is currently licensed for pediatric use. Administration of oseltamivir liquid at a dose of 2 mg/kg per dose twice daily for 5 days was well tolerated and resulted in a 36-hour reduction in the duration of symptoms in children with influenza A.[310] In addition, the use of oseltamivir was associated with a 44% reduction in the frequency of otitis media complicating influenza, and with reductions in antibiotic prescriptions in influenza-infected children. Similarly, therapy of children 5 to 12 years old with symptomatic influenza A and B virus infection who were treated within 36 hours with inhaled zanamivir (10 mg twice a day) resulted in relief of symptoms 1.25 days earlier than did placebo recipients, and a more rapid return to normal activities.[311] Neuraminidase inhibitor therapy of influenza in adults with risk factors for influenza complications has not been evaluated extensively. However, both drugs have shown trends toward efficacy in such populations.[308][312] The results of metaanalyses of the pooled data from subsequent phase III studies have indicated that early treatment with inhaled zanamivir is associated with a median reduction of illness of 2.5 days in older adult and high-risk subjects, and a 3-day earlier return to normal activities.[313] In these pooled analyses, early treatment of high-risk adults and older adults resulted in a 43% reduction in the rates of complications requiring antimicrobials. Drug Resistance Because the neuraminidase inhibitors interact with highly conserved residues within the influenza virus neuraminidase, it has been hypothesized that antiviral resistance will be a relatively limited problem. In fact, truly resistant viruses have been infrequently isolated from immunologically intact individuals treated with neuraminidase inhibitors in clinical trials to date.[314][315] Viruses with reduced susceptibility to oseltamivir have been isolated from 1% of adult and 5.5% of pediatric recipients.[316] Resistant viruses have been recovered more commonly from immunosuppressed children.[217] Viruses resistant to the in vitro antiviral activity of these agents have been isolated after passage in cell culture. Analysis of these viruses has revealed two basic mechanisms of resistance, and it illustrates the interactive roles of the viral HA and NA in binding to and release from infected cells. Mutations within the catalytic framework of the NA that abolish binding of the drugs have been described.[317][318] Depending on the location of the mutation, these viruses may be specifically resistant to only one inhibitor. Resistance mutations in the NA may be associated with altered characteristics of the enzyme with significantly reduced activity.[319][320] A second type of mutation associated with cell cultured resistant viruses involves mutations in the receptor binding region of the hemagglutinin. HA mutations associated with resistance to neuraminidase inhibitors reduce the affinity of the HA for its receptor, allowing cell-to-cell spread of virus in the absence of NA activity.[317][321] It is even possible to generate inhibitor-dependent viruses, in which the affinity for the receptor is apparently so low that NA activity must be inhibited to allow the virus to bind at all. Resistant viruses with HA mutations exhibit cross-resistance to these drugs in cell culture but may retain susceptibility in animal models. Many of these viruses also exhibit reduced virulence in animals. As expected, resistant viruses appear to have significantly reduced fitness, with reduced levels of replication, attenuation in animals, and reduced ability to be transmitted from animal to animal.[322][323][324] These characteristics probably contribute to the relatively low frequency with which these viruses are detected clinically. Treatment of Complications Supportive care, including fluid and electrolyte management, is important. Supplemental oxygen, intubation, tracheotomy, assisted ventilation, and the use of positive end-expiratory pressure may have a role depending on the severity of the illness.[325] For patients with proven or suspected bacterial supra-infection, appropriate antibiotics for the specific organism should be administered. Because of the rapidly advancing nature of many cases of pneumonia occurring during an influenza epidemic, therapy to cover the potential pathogens, including S. pneumoniae and H. influenzae, and possibly S. aureus, is indicated if an etiologic diagnosis cannot be made from a Gram stain of the sputum. There have been no controlled studies of antiviral therapy for the treatment of influenza viral pneumonia, so their use for this condition is based on extrapolation from anecdotal case reports of benefit and on data indicating an effect of amantadine on peripheral airways resistance in uncomplicated influenza.[112][270] In a small controlled study, there was no difference in outcome between hospitalized adults treated with the combination of rimantadine and zanamivir and those treated with rimantadine alone, although both drugs were well tolerated.[326] PREVENTION Vaccines Inactivated Influenza Vaccine The most effective measure available for the control of influenza is the annual administration of inactivated influenza vaccines. Chemically inactivated influenza virus vaccines were first licensed in the United States in 1943. The original vaccine, which consisted of formalin-inactivated whole virions grown in embryonated chicken eggs, was demonstrated to have a protective efficacy of 70% in healthy adults.[11] Since then, although there have been several important advances in the techniques for producing vaccine, the basic vaccine strategy has remained the same. The development of the zonal gradient centrifuge allowed more efficient production and more highly purified vaccines from which reactogenic contaminants had been removed.[327] Treatment of the whole virus with solvents to create “split” vaccines, or with detergents to create “subunit” vaccines, has resulted in a vaccine with fewer adverse reactions, particularly fever, than the whole-cell vaccine.[328] The efficiency of vaccine production has also been improved through the development of techniques to create so called high-yield reassortant strains adapted to grow in high yield from hens’ eggs.[329] The current vaccine is generally formulated as a trivalent preparation, containing one example each of influenza A (H1N1) virus, A (H3N2) virus, and influenza B virus thought to be most likely to cause disease in the upcoming season on the basis of epidemiologic and antigenic analysis of currently circulating strains. Since the late 1970s, the vaccine has been standardized to contain at least 15 μg of each hemagglutinin (HA) antigen as assessed by single radial immunodiffusion (SRID).[330] Safety. Influenza vaccine is generally very well tolerated in adults. Rates of mild local soreness after administration of inactivated influenza vaccine have been documented to be in the range of 60% to 80% in multiple studies.[331][332][333][334] Local side effects are slightly more common in women than in men.[331] Systemic reactions, including malaise, flulike illnesses, and fever, are relatively uncommon. Rates of transient, low-grade fever have varied from 2% to 10% of recipients in these studies; these rates are only marginally increased above the rates in placebo recipients.[331][335] Although whole-virus and split-product vaccines are similarly reactogenic in adults,[336] whole-virus vaccines are associated with fever in children[337] and are no longer available in the United States. Fever occurs in approximately 8% to 11% of vaccinated children and may be associated with other systemic symptoms such as myalgia, arthralgia, headache, and malaise.[338] Severe, life-threatening, immediate hypersensitivity reactions to parenteral inactivated vaccine have been rare. However, hypersensitivity to hens’ eggs, in which the vaccine virus is grown, is a contraindication to vaccination. Generally, if persons can eat eggs or egg-containing products, vaccination is safe. Although vaccine is usually not administered to patients with a genuine anaphylactic hypersensitivity to egg products, such individuals can be desensitized and safely vaccinated if necessary.[339][340][341] During the 1976 National Immunization Program against swine influenza, 45 million persons received influenza vaccine. In the first 4 to 6 weeks after vaccination, the incidence of Guillain-Barré syndrome (GBS) among vaccinees exceeded that among persons who did not receive the vaccine.[342] The estimated risk of acquiring GBS during that vaccination program was 1 in 100,000 vaccinations; the mortality for those with GBS was 5% (i.e., 1 in 2,000,000 vaccinations), and another 5% to 10% had some residual neurologic abnormality.[342] The relationship between inactivated influenza vaccines other than the Swine/New Jersey/76 vaccine and GBS is less clear-cut. National surveillance conducted since 1976 has generally not identified increased rates of this syndrome after vaccination.[343] However, very slight increases in the risk of GBS were seen after the 1992–1993 and 1993–1994 vaccines, representing an excess of approximately one case per million persons vaccinated.[344] Immune Response. Increases in HAI antibody are seen in about 90% of healthy adult recipients of vaccine.[336][345][346] Only a single dose of vaccine is required in individuals who were previously vaccinated or who experienced prior infection with a related subtype, but a two-dose schedule is required in unprimed individuals.[337][347] Primed individuals generally respond with antibody that recognizes a broader range of antigenic variants than do unprimed individuals.[348] Serum antibodies peak between 2 and 4 months after vaccination but fall quickly, reaching near baseline before the next influenza season.[349] Mucosal anti-influenza antibodies are not generated efficiently by parenteral inactivated influenza vaccine.[350][351] Cytotoxic T-lymphocyte or cellular immune responses have been reported after administration of parenteral inactivated influenza vaccine to individuals primed by previous infection.[352] It has recently been reported that human leukocyte antigen (HLA) type is significantly associated with influenza vaccine responsiveness.[353] Groups of adults with potentially decreased responses to inactivated influenza vaccine include older adults,[354][355][356] individuals on immunosuppressive therapy,[357] those with renal disease,[358] and some transplant [359][360][361][362] recipients. To be maximally effective, immunizations should be given before transplantation, should avoid the nadir of white counts, and should include vaccination of close contacts.[363] The responsiveness to influenza vaccination in HIV-infected individuals is related to the degree of immunosuppression.[364][365] Most patients with chronic lung disease respond reasonably well to vaccination, and steroids at doses commonly used to treat reactive airways disease do not appear to preclude vaccine responses.[366][367] Efficacy and Effectiveness. Inactivated influenza vaccine has been shown to be effective in the prevention of influenza A in controlled studies conducted in young adults, with levels of protection of 70% to 90% when there is a good antigenic match between the vaccine and the epidemic virus.[150][368][369] In a recent randomized controlled trial,[370] the efficacy of trivalent inactivated influenza vaccine (TIV) for preventing culture-proven influenza A illness in adults was 76% (95% confidence interval [CI], 58% to 87%) for H1N1 and 74% (95% CI, 52% to 86%) for H3N2. A subanalysis of efficacy in children in this trial demonstrated efficacy of 91% and 77% in preventing symptomatic, culture-positive influenza A H1N1 and A H3N2 illness, respectively, compared with placebo.[371] Vaccination of working adults is also associated with decreased absenteeism from work or school and is significantly cost effective,[372] but these benefits do not extend to years when there is not a good match between vaccine and circulating viruses.[373] In children, TIV has reduced the rates of otitis media in some,[374][375] but not all,[376] studies. Relatively few prospective trials of protective efficacy have been conducted in high-risk populations. In one placebo-controlled prospective trial in an older adult population, inactivated vaccine was approximately 58% effective in preventing laboratory-documented influenza.[377] In addition, numerous retrospective casecontrol studies are available that have documented the effectiveness of inactivated influenza vaccines in older adults.[24][378][379][380][381][382] Vaccine is protective against influenza- and pneumonia-related hospitalization in older adults, and it is accompanied by a decrease in all-cause mortality.[383] Vaccine has also been shown to be protective in limited studies in other high-risk groups, including those with HIV infection.[384] It has recently been shown that inactivated vaccine administered to older adults and persons with coronary artery disease can reduce the rates of coronary events or stroke during the influenza season.[385][386] Live-Attenuated (Cold-Adapted) Influenza Vaccine Recently, the first live-attenuated influenza vaccines for use in humans, the coldadapted influenza vaccine–trivalent (CAIV-T), was licensed for use in the United States in the age group from 5 to 49 years. The use of live-attenuated viruses as influenza vaccines offers several potential advantages over parenteral inactivated vaccines, including induction of a mucosal immune response that closely mimics the response induced by natural influenza virus infection.[387] In addition, the potential superiority of such vaccines in protection of the upper respiratory tract[388] might be useful in strategies using vaccine to limit transmission of influenza. In practical terms, the use of the nasal, rather than the parenteral, route of administration might be more acceptable to patients, particularly in certain age groups. Development of these vaccines takes advantage of the principle of reassortment to generate rapidly attenuated vaccines for new antigenic variants ( Fig. 162–11 ).[389][390] In this case, the master vaccine viruses are the cold-adapted influenza A/Ann Arbor/6/60 (H2N2) and B/Ann Arbor/1/66 viruses, developed by Dr. John Maassab at the University of Michigan in the 1960s.[391] The process of cold adaptation is the repetitive passage of a virus at gradually decreasing temperature until a virus is isolated that replicates efficiently at a low temperature at which the replication of the original wild-type virus is significantly restricted.[392] Figure 162-11 Genetic reassortment is used to generate new liveattenuated vaccine viruses. The genetic basis of attenuation of the “master donor virus” is encoded in gene segments other than the hemagglutinin (HA) or neuraminidase (NA). Using either mixed infection in cell culture or reverse genetics techniques, the genes encoding the HA and NA of new antigenic variants can be inserted into the background of the master donor virus to rapidly create a new attenuated vaccine virus. Genetic analysis of the cold-adapted A/Ann Arbor/6/60 virus has demonstrated multiple mutations in all six of the so-called internal, or non-HA or NA, gene segments, and analysis of single gene reassortants has shown that at least three of these gene segments (PB1, PB2, and PA) participate in the attenuation of the virus in animals and humans.[393][394] Recent studies have also implicated the NP gene in this phenotype.[395] The basis of attenuation of the B/Ann Arbor/1/66 virus has been worked out less completely. Mutations in five of the six internal gene segments have been described,[396] and analysis of laboratory-derived revertant viruses has implicated the PA gene segment as playing an important role in attenuation.[397][398] Safety. CAIV-T or closely related formulations of CAIV have been well tolerated in adults,[370][399][400][401][402][403] with rates of mild nasal symptoms (runny nose, nasal congestion, or coryza) and sore throat occurring at rates slightly in excess of those in placebo recipients. These vaccines have also been shown to be safe and well tolerated in children,[371][404][405][406][407][408][409][410][411][412][413][414] although children under 8 years have had slightly increased but variable rates of low-grade fever, runny nose, and abdominal symptoms in the 7 days after vaccination compared with placebo recipients. However, when considering all the pediatric studies in aggregate, no consistent symptom was significantly more common in CAIV recipients compared with placebo recipients. In older children, 11 to less than 16 years of age, sore throat was observed slightly more frequently in CAIV recipients than in recipients of inactivated influenza.[371] Safety has also been demonstrated in high-risk individuals who would not be able to tolerate even minor lower respiratory tract inflammation. No significant vaccinerelated adverse events were seen in studies of children with cystic fibrosis[415][416] or asthma,[417][418] and vaccinated children with asthma did not experience significant changes in FEV1, use of beta-adrenergic rescue medications or asthma symptom scores compared with placebo recipients.[418] CAIV has also been well tolerated in adults with chronic obstructive airway disease.[419][420][421] Vaccine is very well tolerated in older adults, although in one study vaccine recipients had a 13% excess of sore throats compared with those who received placebo.[399] Young children with advanced HIV infection were reported to have difficulty clearing wild-type influenza virus from the respiratory tract, and there have been several reports of very prolonged virus shedding in highly immunosuppressed individuals,[215] including children with acquired immunodeficiency syndrome (AIDS). However, in small studies in adults[400] and children[408] with HIV who did not have manifestations of AIDS, CAIV-T was well tolerated and not associated with prolonged shedding. Shedding of CAIV does occur in vaccinated adults and particularly in children. Therefore, it is possible that live CAIV viruses could be transmitted to susceptible contacts. However, this does not appear to happen frequently. No transmission of CAIV from vaccine recipients to susceptible contacts was detected in studies of young children involved in daycare-like settings where CAIV and placebo recipients played together for up to 8 hours a day for 7 to 10 days after vaccination.[390][422] In the largest study, 197 children between 8 and 36 months of age in a daycare setting were randomized to receive trivalent CAIV or placebo, and CAIV was detected in one placebo recipient; thus, the estimates of transmissibility in this age group were 0.6% to 2.0%.[423] Importantly, samples of vaccine virus recovered from vaccinated volunteer subjects have all retained the attenuated phenotype and genotype.[423][424] Administration of CAIV to health care workers is not recommended because of potential transmission of virus to patients. The high cost of the vaccine has also restricted its use in institutional settings. Immune Response. Studies of the immunogenicity of cold-adapted reassortant vaccines have been carried out in children, adults, and older adults. The results of these studies are consistent with the hypothesis that the replication of cold-adapted vaccines in the upper respiratory tract, and hence their immunogenicity, is influenced by the susceptibility of the host at the time of vaccination. The frequency and magnitude of immune responses to vaccination are therefore highest in young children, intermediate in adults, and lowest in older adult subjects who have been repeatedly infected with influenza viruses throughout their lifetime. In addition, the mucosally administered CAIV is generally more effective than parenterally administered inactivated influenza vaccine at inducing nasal HA-specific IgA, whereas inactivated vaccine usually induces higher serum titers of HAI and HA-specific IgG antibody.[425] Most susceptible children demonstrate measurable serum and mucosal HA-specific antibody responses.[306][307][409][410][412][414][416][426] Mucosal responses have been demonstrated in up to 85% of young children after CAIV-T.[427] In contrast, adults generally have a low rate of serum antibody response after CAIV,[370][402][403] and relatively lower rates of mucosal responses.[428] Even in those prescreened to have low prevaccination vaccine-specific influenza antibody, the rates of serum antibody responses to intranasal CAIV in adults and older adults are low.[403][429] However, the significance of these findings is unclear, as protection can be demonstrated in some circumstances in the absence of detectable mucosal responses,[430] and the specific levels of mucosal antibody required for protection are unknown. Although not studied extensively, limited data suggest that cold-adapted influenza vaccines may induce antibody and cytotoxic T cells with more broadened recognition within a subtype than seen after inactivated vaccine.[431][432] However, these responses have been more difficult to measure in young children.[433] Efficacy and Effectiveness. CAIV-T was demonstrated to be efficacious in the prevention of influenza in a 2year, randomized, placebo-controlled trial conducted in 1314 children 15 to 74 months of age. Efficacy against culture-confirmed influenza illness in the first year of this trial was 95% against influenza A/H3N2 and 91% against influenza B. In the second year of the trial, the H3 component of the vaccine (A/Wuhan/93) was not a close match with the predominant H3 virus that season, A/Sydney/95. However, the efficacy of CAIV against this variant was 86% (95% CI, 75% to 92%),[405] suggesting that CAIV can induce protective immunity against drift variants. The efficacy in children is also supported by smaller trials using bivalent preparations of CAIV-T.[371][412] Efficacy of CAIV-T against naturally acquired influenza in adults has not been demonstrated directly. However, its efficacy was demonstrated in an experimental infection study in which adults were given either trivalent live intranasal CAIV, parenteral trivalent inactivated influenza vaccine, or placebo, and then experimentally infected with wild-type influenza A/H1N1, A/H3N2, or B virus.[403] The combined efficacy of CAIV-T in preventing laboratory-documented influenza illness was 85%, consistent with observations from other experimental infection studies conducted with monovalent CAIV.[161][388][434][435] In addition, in a large, 5year field trial in Nashville, Tennessee,[370] the efficacy of bivalent CAIV was 85% (95% CI, 70% to 92%) against A/H1N1 illness and 58% (95% CI, 29%-75%) against A/H3N2 illness. Use of CAIV-T in adults has also been shown to reduce rates of severe febrile illness of any cause during the influenza season.[401] No studies of the protective efficacy of CAIV alone have been conducted in older adults because of the possibly reduced immunogenicity of the vaccine in this age group. However, the combination of local live-attenuated influenza vaccine and parenteral inactivated vaccine administered together was shown to result in an approximately 60% decrease in cases of laboratory-confirmed influenza in an older adult nursing home population, compared with inactivated vaccine alone.[436] Recommendations for Vaccine Use The main goal of the strategy for use of influenza vaccine is to reduce complications by targeting vaccine to those individuals at highest risk of influenzarelated hospitalizations or death. Table 162–6 lists those groups for whom annual influenza vaccination is currently recommended,[437] including older adults and adults and children with chronic conditions known to increase the risk of influenza complications. The age at which annual vaccination is recommended has been lowered from 65 to 50. The rationale for this recommendation is to achieve higher vaccination rates in adults with high-risk conditions, a large proportion of whom are between 50 and 65 years old. Table 162-6 -- Groups Targeted for Influenza Immunization Persons at Increased Risk for Complications • Persons aged ≥65 years • Residents of nursing homes and other chronic-care facilities • Adults and children with chronic pulmonary or cardiovascular diseases, including asthma • Adults and children with chronic metabolic diseases (including diabetes mellitus), renal dysfunction, hemoglobinopathies, or immunosuppression (including HIV) • Children and adolescents receiving long-term aspirin therapy • Women who will be in the second or third trimester of pregnancy during the influenza season • Children aged 6 mo-23 mo Persons Aged 50–64 Years • Recommended for this entire age group to increase vaccination rates among persons in this age group with high-risk conditions Persons Who Can Transmit Influenza to Those at High Risk • Physicians, nurses, and other personnel in both hospital and outpatient-care settings, including medical emergency response workers • Employees of nursing homes and assisted living and other chronic-care facilities who have contact with patients or residents • Persons who provide home care to persons in groups at high risk • Household contacts (including children) of persons in groups at high risk • Household contacts of children aged 0–23 months Recommendations for annual vaccination of healthy children are also being considered. The Advisory Committee on Immunization Practices (ACIP) has recommended that practitioners vaccinate all children 6 to 23 months of age with the influenza vaccine,[437] because of the high rates of influenza-related hospitalizations and medically attended illness in this age group. An additional benefit of widespread vaccination of young children could be reductions in rates of influenza in other groups, because children play an important role in the propagation of influenza epidemics in a community.[438] Relatively little direct evidence supports the use of influenza vaccine to prevent transmission, but in one study, mass vaccination of school-aged children resulted in reduced rates of influenza in teachers and parents compared with a control community where children were not vaccinated.[439] Vaccination of children in daycare has been reported to reduce the rates of febrile respiratory illnesses in unvaccinated household contacts.[440] In addition, it has been observed that influenza-related mortality rates among older adults have increased in Japan, coincident with discontinuation of that country’s policy of universal vaccination of school children.[441] Such observations suggest that expanding the population of children targeted for annual influenza immunization could be a reasonable approach to reducing the impact of influenza in the whole community. For similar reasons, vaccination of individuals who are in close contact with persons with high-risk conditions is strongly recommended, including health care workers. At a minimum, such a policy would reduce workplace absences and prevent disruptions in care.[442] In addition, there is supportive evidence that vaccination of health care workers reduces mortality in patients, at least among residents of nursing homes, independently of the vaccination status of the patients themselves.[443][444] Except for the influenza pandemics of 1918–1919 and 1957–1958, influenza in pregnancy has not been associated with increased mortality or fetal loss.[445] However, the increased physiologic demands of pregnancy could be associated with enhanced severity of influenza, and recent studies suggest that there is a significant increase in hospitalizations for cardiorespiratory conditions among women in the third trimester of pregnancy during influenza season.[29] There has been a considerable experience with the use of influenza vaccine in pregnancy, and it appears to be safe in this situation. Therefore, current recommendations are to administer vaccine to women who will be in the second or third trimester (i.e., at >14 weeks’ gestation) during influenza season.[446] A secondary benefit of this strategy could be the provision of antibody to the infant, depending on the timing of maternal immunization.[447] Because of the high rate of spontaneous fetal loss during the first trimester, vaccination should generally be avoided during this period, unless the pregnant woman has other high-risk medical conditions, in which case vaccine should be administered regardless of the stage of pregnancy. The duration of protective immunity appears to be limited, particularly in older adults,[448] and in most years, one or more of the vaccine components are updated to keep pace with antigenic drift in circulating viruses. Thus, current inactivated vaccines must be administered yearly. In some situations, yearly administration has been reported to result in decreased effectiveness.[449] Recent studies suggest that prior immunization does not adversely affect immune responses to vaccination or the protection afforded by inactivated vaccine, at least in healthy adults.[450] Chemoprophylaxis All four of the available antiviral agents are effective at preventing influenza prophylaxis, provided drug is administered continuously throughout the period of exposure. Several schemes for such prophylaxis have been evaluated, including seasonal prophylaxis, where drug is administered throughout the influenza epidemic season, generally 4 to 6 weeks; family prophylaxis, where drug is administered to family members for a short period of time after recognition of an index case in the family; and outbreak-initiated prophylaxis in institutions, which could be considered to be a variation on the theme of family prophylaxis. In addition, short-term antiviral prophylaxis can be considered for high-risk individuals who are vaccinated during the influenza season. Seasonal Prophylaxis Seasonal prophylaxis with amantadine has been shown to result in protection rates of 70%[451] to 90%[271] against H1N1 viruses, and 68% against H3N2 viruses.[452] Seasonal prophylaxis with amantadine has also been effective in children, in whom an approximately 90% reduction in laboratory-confirmed illness due to influenza A H2N2 was reported.[453][454] Significantly fewer studies of prophylaxis with rimantadine have been performed. However, when rimantadine and amantadine were directly compared in seasonal prophylaxis in healthy adults, the levels of protection were approximately equal.[271] Both zanamivir and oseltamivir have also been shown to be protective in seasonal prophylaxis. In healthy adults, inhaled zanamivir was shown to have about 67% efficacy for prevention of confirmed influenza,[455] and in a similar study, the efficacy of oral oseltamivir was 74%.[456] Both drugs were well tolerated on prolonged use. However, only oseltamivir is approved for prophylaxis (for individuals 2 and 3 years of age.) Relatively less information is available about the use of any of the influenza antivirals for prophylaxis in older adult or high-risk populations. In one study, seasonal prophylaxis was highly effective in preventing laboratory-documented influenza in older adult residents of retirement communities.[457] Importantly, 80% of the subjects had previously been vaccinated, and prophylaxis resulted in a 91% reduction in influenza in this group. Thus, vaccine and chemoprophylaxis had an additive protective effect in older adults. Family Prophylaxis Results of outbreak prophylaxis in the family setting have yielded conflicting results depending on whether the index case does or does not receive concurrent therapy. When the index case was not treated with amantadine, protection of family contacts was seen for both drugs.[458][459][460] However, if the index case was treated with amantadine at the same time that contacts received prophylaxis, no protection was seen,[287][461] presumably because of the generation and transmission of resistant virus in this setting.[456] In contrast, use of oseltamivir[462] or zanamivir[463] is associated with 80% protection without the development or transmission of resistant virus. Generally, drug is administered to contacts for 5 to 7 days after recognition of the index case. It is important to realize that treated individuals remain susceptible to infection from outside the family after such prophylaxis is discontinued. Outbreak Prophylaxis Probably one of the most common uses of antiviral agents for influenza is to terminate the transmission of influenza within institutions such as nursing homes during outbreaks. Although this has not been subject to formal, placebo-controlled study, many anecdotal reports support the efficacy of amantadine,[273][464][465] zanamivir,[466] and oseltamivir[467][468] in this setting. When M2 inhibitors are used for outbreak prophylaxis, individuals who are receiving treatment with amantadine should be isolated from those who are receiving prophylaxis. Failure to adhere to this practice is associated with the development and transmission of resistant viruses within the institution.[290][469] One preliminary report has suggested that prophylactic administration of zanamivir was successful in terminating an outbreak of influenza in a nursing home in which cases continued to occur despite amantadine prophylaxis.[470]