Outpt Immunosuppression
advertisement

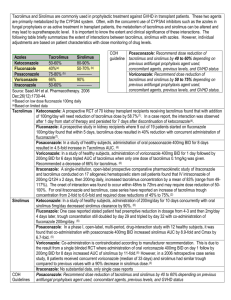
Policy: Post-Liver Transplant Outpatient Immunosuppression Protocol Statement: 1. Activation date: 4/28/08 2. Affected Department: Liver Transplant Program 3. Vision Strategy: Patient care 4. Policy Statement: The Emory Transplant Center will comply with all applicable federal, state and local laws, regulations and policies regarding the management of prescribing medications and refills. 5. Basis: This policy is necessary for the protection of patients, physicians and staff. 6. Administrative Responsibility: Section heads, physicians, practitioners and staff are responsible for compliance with this policy. Scope/Procedure: 1. Tacrolimus (Prograf) a. Tacrolimus is the preferred initial agent utilized in post-liver transplant immunosuppression. Dosage will be adjusted based on disease process and serum tacrolimus levels. Tacrolimus dosage should be decreased in the presence of adverse events as clinically warranted. Dosage adjustments should be balanced between severity and timing of rejection episodes in relation to date of transplant. b. In the first 3 months post-transplant, tacrolimus levels should be targeted to 812ng/ml. See the chart below for other goal trough levels. Trough level goals should be individualized to the patient. c. Tacrolimus conversion to other immunosuppressive agents may be considered if toxicities persist. Dosing of any additional immunosuppressive agents will be individualized to a particular patient depending upon the clinical events leading to conversion. Reasons to convert from tacrolimus to a different immunosuppressive agent (such as sirolimus or cyclosporine) include the following: i. Nephrotoxicity ii. GI toxicity iii. Neurotoxicity not responsive to reasonable attempts at dosage adjustments or other intervention iv. Hyperglycemia not responsive to dosage adjustments or treatment with oral antidiabetic agents v. Hemolytic Uremic Syndrome (HUS) vi. Severe alopecia 2. Cyclosporine (Gengraf) a. Cyclosporine is often utilized in patients who do not tolerate tacrolimus (Prograf) well (see Section 1b above). Dosage should be individualized for each patient based on disease process and serum cyclosporine levels. Cyclosporine dosage should be decreased in the presence of adverse events as clinically warranted. Dosage adjustments should be balanced between severity and timing of rejection episodes in relation to date of transplant. b. Dose adjustment of cyclosporine or conversion to another immunosuppressive agent such as sirolimus (Rapamune) should be considered for adverse events such as gingival hyperplasia, persistent hypertension, excessive growth of hair and nephrotoxicity. c. Patients newly started on cyclosporine should be started on modified cyclosporine (Gengraf® or Neoral ®) equivalent formula of cyclosporine. Non-modified cyclosporine (Sandimmune) should not be used for patients starting on cyclosporine. d. In the first 3 months post-transplant, cyclosporine levels should be targeted 150180. See the chart below for other goal trough levels. Trough level goals should be individualized to the patient. 3. Mycophenolate mofetil (CellCept) a. Mycophenolate mofetil (MMF or CellCept) can be used as part of a dual or triple immunosuppressive regimen. MMF will be dosed at 1gram Q12hr initially posttransplant. b. MMF should be reduced in the following situations: i. WBC < 4 1. Decrease dose by 50% 2. Obtain differential ii. WBC < 2 1.Hold MMF 2. Obtain differential iii. ANC < 1000 1. Hold MMF 2. Consider filgastrim 300mcg SQ iv. Severe diarrhea/nausea/vomiting 1. Change interval to four times daily, maintaining current dose 2. Decrease dose by 50% v. Severe Infection 1. Decrease dose by 50% c. Reasons to discontinue MMF early include the following: i. Bone marrow suppression not responsive to dose reduction ii. GI toxicity not responsive to dose interval changes (i.e. 4 times daily) or dosage reduction (i.e. 500mg BID) ii. Severe infection d. Dosage may be increased to 1500mg BID at the discretion of the transplant team for higher immunosuppressive levels. e. Myfortic (mycophenolic acid) i. Mycophenolic acid may be considered as an alternative to MMF for those patients that continue to have GI intolerance to MMF or do not tolerate the dose reduction of MMF ii. Dosing: MMF 1000mg po BID = Myfortic 720mg po BID Myfortic is available in 180mg and 360mg tablets 4. Sirolimus (Rapamune) a. Sirolimus is usually not utilized until 1 month post-transplant due to the potential risk for delayed wound healing and of hepatic artery thrombosis. It may be used as a substitute for tacrolimus in the event of persistent renal insufficiency (see Renal Sparing Protocol). If sirolimus is utilized it will be at an initial dose of 2 -3 mg per day. Doses should be individualized for each patient based on disease process and serum sirolimus levels. Sirolimus conversion should be considered if a patient has a serum creatinine consistently above 1.2. b. Conversion to a calcineurin inhibitor (CNI) should be considered in any patient on sirolimus undergoing a surgical operation, such as a hernia repair, prior to the scheduled surgical date to help promote better wound healing post procedure. Patients may be converted back to sirolimus after the wound has healed. 5. Everolimus (Zortress) a. Everolimus may be considered in patients 30 days or more from transplant. Everolimus may be considered as an alternative to CNIs or sirolimus for patients with renal insufficiency (see Renal Sparing Protocol). Everolimus may also have beneficial anti-tumor effects as well and may have a role in patients with skin cancer, HCC, renal cell carcinoma or other malignancies. b. Usual starting dose of everolimus is 1-1.5mg po q12hr. Initial goal trough levels should range 8-12ng/ml. Everolimus is not recommended as monotherapy due to a potential increase in acute cellular rejection. c. Prior to initiating everolimus, the following laboratory values should be obtained: spot urine protein, baseline fasting glucose, HbgA1c, fasting lipid profile with triglycerides. The wound should be healed prior to initiation of everolimus. d. Side effects of everolimus include, but are not limited to: proteinuria, hypertriglyceridemia, pancytopenia, delayed wound healing, edema, mouth ulcers, new onset diabetes, male infertility. e. Conversion to a CNI should be considered in any patient on everolimus undergoing a surgical operation, such as a hernia repair, prior to the scheduled surgical date to help promote better would healing post procedure. Patients may be converted back to everolimus after the wound is healed. 6. Steroids (prednisone) a. Steroids may be used as part of the initial post-transplant induction phase (see Inpatient Immunosuppressive Protocol). b. Patients are to be weaned off steroids based on their primary disease and freedom from rejection. Weaning of steroids is based entirely on the clinical presentation of patients. Patients with autoimmune hepatitis (AIH) may not benefit from steroid weans and may be maintained on 5-10mg prednisone daily. 7. Use of generic immunosuppression a. The use of generic immunosuppression is allowed. The transplant team encourages patients to take generics from the same manufacturer, when possible, to minimize potential affects on trough levels. Immunosuppressive Levels and Steroid Taper Drug 0-3 months 6-12 3-6 months months Tacrolimus (Prograf) levels 8-12 levels 6-12 levels 4-10 levels 3-8 Cyclosporine (Gengraf) levels 100- levels 75- levels 50125 100 levels 150-180 150 MMF (Cellcept) 1000mg 1000mg BID BID Sirolimus (Rapamune) or Everolimus (Zortress) 1000mg BID > 1 year 1000mg BID 1 m levels 6-12 levels 4-10 levels 3-8 o n t h 8 1 2 Prednisone 10 days 1 month 2 months 3 months 6 months Non-Viral Hepatitis, HBV/Others, PSC, PBC 20mg QAM Off at day 10mg QAM 5mg QAM 90 off AIH 20mg QAM 10mg QAM 5 mg QAM 5mg 5mg > 1 year 5mg Immunosuppressive regimens will be individualized on a case by case basis based on creatinine, serum potassiuim, liver function profile, history of rejection or CMV status, and underlying disease process. References: Simone PD, Nevens F, DeCarlis L, et al. Everolimus with reduced tacrolimus improves renal function in de novo liver transplant recipients: a randomized controlled trial. AJT 2012; 12: 3008-3020. Fisher L, Klempnauer J, Beckebaum S, et al. A randomized, controlled study to assess the conversion from Calcineurin inhibitors to everolimus after liver transplantation – PROTECT. AJT 2012;12: 1855-1865. Masetti M, Montalti R, Rompianesi, et al. Early withdrawal of Calcineurin inhibitors and everolimus monotherapy in de novo liver transplant recipients preserves renal function. AJT 2010;10: 2252-2262. Approved by: Liver Transplant Leadership Group __________________________________ Stuart J. Knechtle, M.D. Chair, Liver Transplant Leadership Group Director, Liver Transplant Program ____________________________________ James Spivey, MD Medical Director, Liver Transplant Program Approval Dates: 4/28/08, 3/8/10, 4/2011, 5/13/2011, 7/2013, 6/24/2014