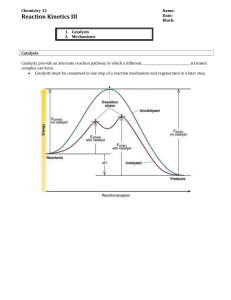
Kinetics Problem Set 3 – Mechanisms AP Chemistry – 2015/2016 Name ________________________________________ Period ______ Date ______________ (12 points) 1. Answer the following questions regarding mechanisms: a. What is meant by the term molecularity? 1 point b. Why are termolecular elementary reactions so rare? 1 point c. What is an intermediate in a mechanism? 1 point 2. Write possible rate laws for the following elementary reactions: 2 points a. CH3NC (g) CH3CN (g) b. O3 (g) + NO (g) O2 (g) + NO2 (g) c. O3 (g) + O (g) 2 O2 (g) 3. A proposed mechanism for a reaction is: C4H9Br C4H9+ + Br + C4H9 + H2O C4H9OH2+ 2 points C4H9OH2+ + H2O C4H9OH + H3O+ slow fast fast a. Write the rate law expected for this mechanism. b. What is the overall balanced equation for the reaction? c. What are the intermediates in the proposed mechanism? 4. The following mechanism has been proposed for the gas-phase reaction of H2 with ICl: H2 (g)+ ICl (g) HI (g) + HCl (g) 2 points HI (g) + ICl (g) I2 (g) + HCl (g) a. Wrote the balanced equation for the overall reaction. b. Identify any intermediates in the mechanism. c. If the first step is slow and the second one is fast, which rate law do you expect to be observed for the overall reaction? 5. You have studied the gas-phase oxidation of HBr by O2: 4 HBr (g) + O2 (g) 2 H2O (g) + 2 Br2 (g) You find the reaction to be first order with respect to HBr and first order with respect to O2. You propose the following mechanism: HBr (g) + O2 (g) HOOBr (g) HOOBr (g) + HBr (g) 2 HOBr (g) 3 points HOBr (g) + HBr (g) H2O (g) + Br2 (g) a. Confirm that the elementary reactions add to give the overall reaction. b. Based on the experimentally determined rate law, which step is rate determining? c. What are the intermediates in this mechanism? d. If you are unable to detect HOBr or HOOBr among the products, does this disprove your mechanism? 6. The reaction 2 NO (g) + Cl2 (g) 2 NOCl (g) was performed and the following data was obtained: 3 points Is the following mechanism consistent with the data? Explain fully. NO (g) + Cl2 (g) NOCl2 (g) NOCl2 (g) + NO (g) 2 NOCl (g)