hazard mitigation in nonclinical safety assessment
advertisement

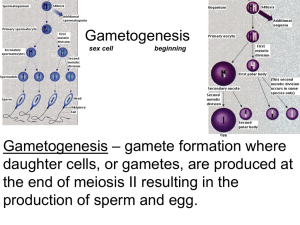
Male Reproductive Pathology: Hazard Characterization and Mitigation in Nonclinical Safety Justin D. Vidal, DVM, PhD, DACVP Director and Head of Pathology Safety Assessment, Upper Merion GlaxoSmithKline justin.2.vidal@gsk.com Introduction Prior to administering the first dose in healthy male volunteers, often the sum total of the assessment on the male reproductive system falls on the study pathologist! According to ICH Harmonized Tripartite Guidelines Detection of Toxicity to Reproduction for Medicinal Products & Toxicity to Male Fertility S5(R2): Information on potential effects on spermatogenesis can be derived from repeated dose toxicity studies and histopathology of the testis has been shown to be the most sensitive method for the detection of effects on spermatogenesis. As such the toxicologic pathologist is required to have a sound understanding of spermatogenesis, stageaware evaluation of the testis, and normal physiology and endocrinology in order to carefully and thoroughly screen and evaluate potential xenobiotic-related effects on the male reproductive system. In addition, the toxicologic pathologist’s role often extends beyond study pathology as this information and understanding is critical to the hazard characterization and mitigation of risk in nonclinical safety. As described in Vidal et al, 2013, several key issues need to be considered before considering the potential impact of a male reproductive finding in a nonclinical study: In general, the regulatory viewpoint is that any detectable, drug-induced change in spermatogenesis or sperm parameters is considered adverse, regardless of whether the change affects fertility in the test species. There is relatively good concordance between animal models and man (e.g. glycol ethers, lead, 1,2- dibromochloropropane, various marketed compounds) Testicular toxicants will often demonstrate species differences between rat and dog, with one being sensitive and the other showing no effect Since spermatogenesis and its regulation are basically similar between mammalian species, neither species is considered more or less relevant to man than the other. NHP is not considered any more relevant to man than rat or dog and regardless of how many species are resistant versus sensitive, risk assessment will generally be based on the most sensitive species. When presented with a new finding in the male reproductive system, a number of questions need to be addressed: Is the finding biologically relevant to humans? Does it impact the clinical protocol or informed consent? Is it patients with advanced disease/limited therapeutic options? Is there a large exposure margin? What is the time course/development of the lesion(s)? Is it reversible? Do you know the mechanism? Can you monitor in the clinic? Feedback from clinicians and regulatory agencies often include questions about reversibility, potential mechanism, and/or clinical monitoring and in many cases additional nonclinical studies are needed. While these questions are very similar to what would be asked for toxicity in any organ system, there are a number of unique features that make working with the male reproductive system a challenge including: the blood testis barrier, duration of spermatogenesis and impact on timing of onset and recovery, and difficulty in clinical monitoring. The approach(s) to addressing these questions in light of these challenges will be reviewed and discussed using case examples of tool compounds. References 1. Abuelhija M, Weng CC, Shetty G, Meistrich ML. 2012. Differences in radiation sensitivity of recovery of spermatogenesis between rat strains. Toxicol Sci. 126(2):54553. 2. Abuelhija M, Weng CC, Shetty G, Meistrich ML. 2013. Rat models of post- rradiation recovery of spermatogenesis: interstrain differences. Andrology. 1(2):206-15. 3. Chapin R.E. 2011. Whither the resolution of testicular toxicity. Birth Defects Res B Dev Reprod Toxicol. 92(6):504-7. 4. Chapin RE, Creasy DM. 2012. Assessment of circulating hormones in regulatory toxicity studies II. Male reproductive hormones. Toxicol Pathol. 40(7):1063-78 5. Clegg, E.D., J.C. Cook, R.E. Chapin, P.M.D. Foster, and G.P. Daston. 1997. Leydig cell hyperplasia and adenoma formation: Mechanisms and relevance to humans. Reprod Toxicol 11:107-21. 6. Clermont, Y. 1972. Kinetics of spermatogenesis in mammals: Seminiferous epithelium cycle and spermatogonial renewal. Physiol Rev 52:198-236. 7. Clifton DK, Bremner WJ. 1983. The effect of testicular x-irradiation on spermatogenesis in man. A comparison with the mouse. J Androl. 4(6):387-92. 8. Cook, J.C., G.R. Klinefelter, J. F. Hardisty, R.M. Sharpe, and P.M.D. Foster. 1999. Rodent Leydig cell tumorigenesis: A review of the physiology, pathology, mechanisms, and relevance to humans. Crit Rev Toxicol 29:169-261. 9. Creasy D.M. 2010. Series on Testing and Assessment: Testing for Endocrine Disruptors. No.106: Guidance document for histopathologic evaluation of endocrine and reproductive tests in rodents. Part 2 and Part 4 http://www.oecd.org/document/30/0,3343,en_2649_34377_1916638_1_1_1_1,00.html. 10. Creasy, D.M. 1997. Evaluation of testicular toxicity in safety evaluation studies: the appropriate use of spermatogenic staging. Toxicol Pathol 25:119-31. 11. Creasy, D.M. 2001. Pathogenesis of male reproductive toxicity. Toxicol Pathol 29:64-76. 12. Creasy, D.M., and P.M.D. Foster. 2002. Male reproductive system. In Handbook of toxicologic pathology, ed. W.M. Haschek, C.G. Rousseaux, and M.A. Wallig, 785-846. San Diego: Academic Press. 13. Ezer, N. and B. Robaire, B. 2002. Androgenic regulation of the structure and function of the epididymis. In The epididymis: from molecules to clinical practice, ed. B. Robaire, and B.T. Hinton, 297-316. New York: Kluwer Academic/Plenum Publishers. 14. Foley, G.L. 2001. Overview of male reproductive pathology. Toxicol Pathol 29:49-63. 15. Hess R.A. 1990. Quantitative and qualitative characteristics of the stages and transitions in the cycle of the rat seminiferous epithelium: Light microscopic observation of perfusion-fixed and plastic embedded testes. Biol. Reprod. 43, 525-542. 16. Hess R.A. 1998. Effects of environmental toxicants on the efferent ducts, epididymis and fertility. J Reprod Fertil Suppl 53:247-259. 17. Kerr , J.B, M. Millar, S. Maddocks, and R.M. Sharpe. 1993. Stage-dependent changes in spermatogenesis and Sertoli cells in relation to onset of spermatogenic failure following withdrawal of testosterone. Anat Rec 235:547-559. 18. Kerr, J.B. 1992. Spontaneous degeneration of germ cells in normal rat testis: assessment of cell types and frequency during the spermatogenetic cycle. J Reprod Fertil 95:825-30. 19. Leblond, C.P., and Y. Clermont. 1952. Definition of the stages of the cycle of the seminiferous epithelium in the rat. Ann N.Y Acad Sci 55:548-73. 20. Lee, K.-P., S.R. Frame, G.P. Sykes, and R. Valentine. 1993. Testicular degeneration and spermatid retention in young male rats. Toxicol Pathol 21:292-302. 21. Mabeck, L. M., M.S. Jensen, G. Toft, M. Thulstrup, M. Andersson, T.K. Jensen, A. Giwercman, J. Olsen, J.P. Bonde, and the Danish First Pregnancy Planners Study Team. 2005. Fecundability according to male serum inhibin B – a prospective study among first pregnancy planners. Human Reprod. 20 (10), 2909-2915. 22. Meachem, S. J., E. Nieschlag, and M. Simoni. 2001. Inhibin B in male reproduction: pathophysiology and clinical relevance. Eur J Endocrinol 145:561-571. 23. Meistrich ML, Shetty G. 2003. Suppression of testosterone stimulates recovery of spermatogenesis after cancer treatment. Int J Androl. 26(3):141-6. 24. Meistrich ML, Wilson G, Porter KL, Huhtaniemi I, Shetty G, Shuttlesworth GA. 2003. Restoration of spermatogenesis in dibromochloropropane (DBCP)-treated rats by hormone suppression. Toxicol Sci. 76(2):418-26. 25. Meistrich, M.L. 1986. Components of testicular function and sensitivity to disruption. Biol Reprod 34:17-28. 26. Morales, A., F. Mohamed, and J.C. Cavicchia. 2007. Apoptosis and blood-testis barrier during the first spermatogenic wave in the pubertal rat. Anat Rec 290:206-14. 27. O’Connor J. C., J.C. Cook, M.S. Marty, L.G. Davis, A.M. Kaplan, and E.W. Carney. 2002. Evaluation of Tier 1 screening approaches for detecting endocrine-active compounds (EACs). Crit Rev Toxicol 32:521-549. 28. Parchuri N, Wilson G, Meistrich ML. 1993. Protection by gonadal steroid hormones against procarbazine-induced damage to spermatogenic function in LBNF1 hybrid rats. J Androl. 14(4):257-66. 29. Rehm, S., T.E. White, E.A. Zahalka, D.J. Stanislaus, R.W. Boyce, and P.J. Weir. 2008. Effects of food restriction on testis and accessory glands in maturing rats. Toxicol Pathol 36:687-94. 30. Russell, L.D., J.P. Malone, and S.L. Karpas. 1981. Morphologic pattern elicited by agents affecting spermatogenesis by disruption of its hormonal stimulation. Tissue and Cell 13:369-380. 31. Russell, L.D., R.A. Ettlin, A.P. SinhaHikim, and E.D. Clegg. 1990. Histological and histopathological evaluation of the testis. Clearwater: Cache River Press. 32. Saito, K., L. O'Donnell, I. McLachlan, and D.M. Robertson. 2000. Spermiation failure is a major contributor to early spermatogenic suppression caused by hormone withdrawal in adult rats. Endocrinology 141:2779-85. 33. Sharpe, R. M. 1994. Regulation of spermatogenesis. In: The Physiology of Reproduction. Second Edition. Knobil E and Neil JD, (Eds.) pp 1363-1434 Raven Press, New York. 34. Shetty G, Wilson G, Huhtaniemi I, Shuttlesworth GA, Reissmann T, Meistrich ML. 2000. Gonadotropin-releasing hormone analogs stimulate and testosterone inhibits the recovery of spermatogenesis in irradiated rats. Endocrinology. 141(5):1735-45. 35. Su L, Mruk DD, Lee WM, Cheng CY. 2011. Drug transporters and blood--testis barrier function. J Endocrinol. 209(3):337-51. 36. Vidal JD, Mirsky ML, Coleman K, Whitney KM, Creasy D. 2013. Reproductive System and Mammary Gland. In: Toxicologic Pathology: Nonclinical Safety Assessment. Eds. Sahota PS, Popp JA, Hardisty JF, and Gopinath C. Taylor Francis. 37. Weinbauer, G.F. and E. Nieschlag. 1999. Testicular physiology of primates. In Reproduction in nonhuman primates, ed. G.F. Weinbauer and R. Korte, 13-26. Münster: Wasmann Verlag. Ziejewski MK, Vidal JD, Stanislaus D, Apostoli A, Chowdhury P, Laffan S. 2013. The inhibin B response to a motilin receptor agonist in male rats. Birth Defects Res B Dev Reprod Toxicol. 98(1):63-71.