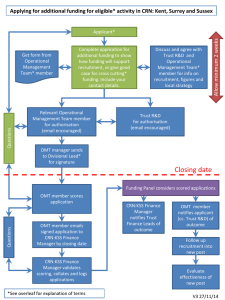
Sequencing request form
advertisement
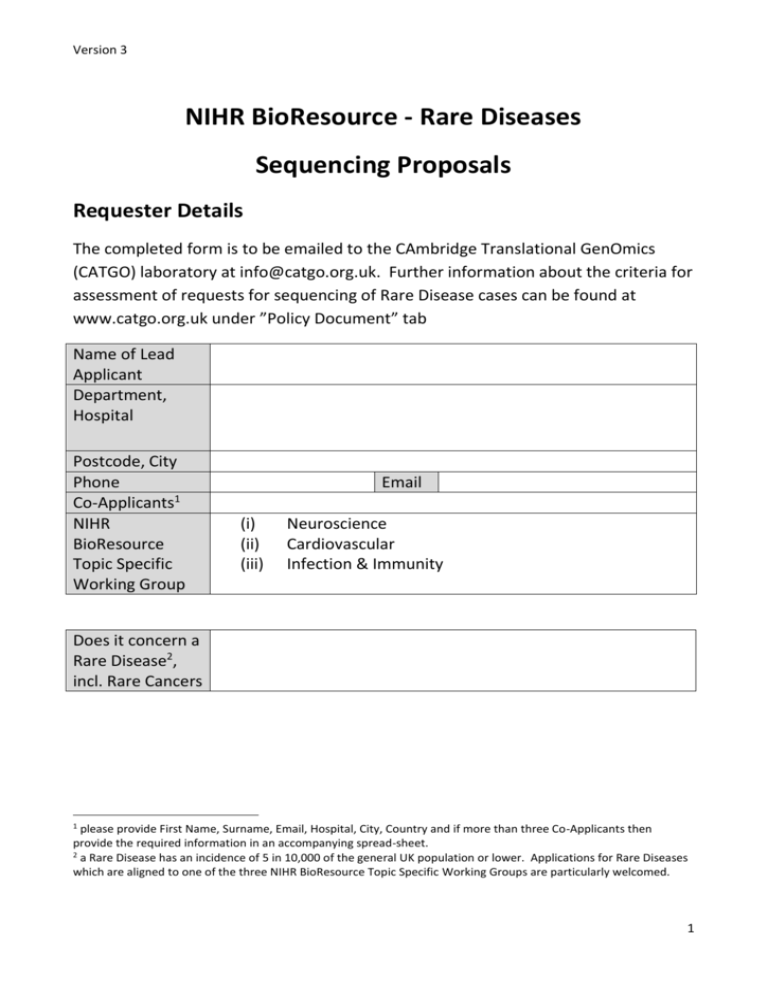
Version 3 NIHR BioResource - Rare Diseases Sequencing Proposals Requester Details The completed form is to be emailed to the CAmbridge Translational GenOmics (CATGO) laboratory at info@catgo.org.uk. Further information about the criteria for assessment of requests for sequencing of Rare Disease cases can be found at www.catgo.org.uk under ”Policy Document” tab Name of Lead Applicant Department, Hospital Postcode, City Phone Co-Applicants1 NIHR BioResource Topic Specific Working Group Email (i) (ii) (iii) Neuroscience Cardiovascular Infection & Immunity Does it concern a Rare Disease2, incl. Rare Cancers 1 please provide First Name, Surname, Email, Hospital, City, Country and if more than three Co-Applicants then provide the required information in an accompanying spread-sheet. 2 a Rare Disease has an incidence of 5 in 10,000 of the general UK population or lower. Applications for Rare Diseases which are aligned to one of the three NIHR BioResource Topic Specific Working Groups are particularly welcomed. 1 Version 2.0 Scientific proposal Background: the following headings need to be addressed in the Background. The overall length of the Background should not exceed 1 side of A4 Clinical phenotype The collection of cases Which underlying genetic architecture is assumed Which power calculations have been performed 2 Version 2.0 Any further comments about the proposed study which need addressing (do not exceed ½ A4) 3 Version 2.0 Analysis plan Will you require bioinformatics support from NIHR BioResource - Rare Diseases? Please outline your proposed analysis plan Who will be providing other bioinformatics/statistical genetics support? Please state name, position and institution. Name Position Institution 4 Version 2.0 Sample details Q1: Number of samples requested Q2: Has ethical approval been obtained for the - (a) sequencing of the coding fraction of the genome (the exome)? - (b) sequencing of the entire genome? - (c) release of the sequencing data on the European Genomephenome Archive under a “Click-It” agreement? Yes / No Yes / No Yes / No Q3: Please indicate the number of samples which may be imported into Cambridge from the beginning of Quarter 2-2013 until the end of Quarter 1-2014. It is essential for the planning of the Exome-seq pipeline to provide reliable figures for sample imports over the next 4 quarters Calendar Quarter -year No. of % of total samples sample collection Quarter 3-2013 Quarter 4-2013 Quarter 1-2014 Quarter 2-2014 Q4: Are samples for which you seek Genome-seq capacity now and in the future from cases of: Centre & Country Number % NIHR Biomedical Research Centres ~ NIHR Biomedical Research Units~ Other UK centres~ Other European centres US centres Centres in other countries not listed above Q5: Can you indicate whether the proposed project will influence patient care in the NHS over the next 5 years; please explain. (do not exceed ½ A4) 5 Version 2.0 Q6: Do you have any other comments on your request (do not exceed ½ A4) ~The priority of the NIHR BioResource Genome-seq is to sequence samples from individuals who have been consented to the NIHR BioResource - Rare Diseases), or in the case of existing collections where there is agreement to seek consent for enrolment in the NIHR BioResource - Rare Diseases in parallel. Some capacity may be available for already collected DNA samples where it is not possible to seek NIHR BioResource - Rare Diseases consent. For expansion of collections enrolment via the NIHR BioResource - Rare Diseases is a prerequisite to get access to the NIHR BioResource Genome-seq capacity. DNA samples from abroad are permissible under certain circumstances, e.g. in the case of Rare Diseases and co-investment from separate 6 Version 2.0 funding streams for a part of the Genome-seq costs from locally available budgets will be agreed. Studies with relevance for one of the three following clinical domains: (i) Neurological Disorders, above all those with relevance to Dementia and Alzheimer’s Disease; (ii) Cardiovascular Diseases, (iii) Infection & Immunity are particularly welcomed. The application will be assessed by the NIHR BioResource - Rare Diseases Sequencing and Informatics Committee. The committee will use the following criteria to assess the application. Application Number:………………………. 7