SO 2 Cl 2 (g)
advertisement

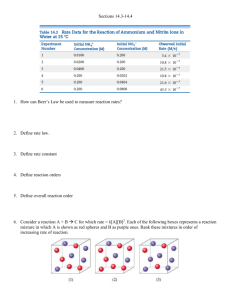
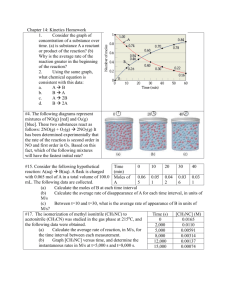
Name _________________________________________________ Date ___________________ Period ____ Homework Chapter 14: Chemical Kinetics Exercises: Sections 14.4: Change of Concentration with Time 1. (a) What is a second-order reaction? (b) For a second-order reaction, what quantity, when graphed versus time, will yield a straight line? (c) How do the half-lives of first-order and second-order reactions differ? 2. The gas-phase decomposition of SO2Cl2, SO2Cl2(g) → SO2(g) + Cl2(g) is first order in SO2Cl2. At 600 K, the half-life for this process is 2.3 x 105 s. (a) What is the rate constant at this temperature? Follow ALL math work rules! (b) At 320oC the rate constant is 2.2 x 10 – 5 s – 1 . What is the half-life at this temperature? Follow ALL math work rules! 1 3. As described in Problem 2 above, the decomposition of sulfuryl chloride, SO2Cl2, is a first-order process. The rate constant for the decomposition at 660 K is 4.5 x 10 – 2 s –1 . NOTE: Sig Figs and LOGS: for log = total # of digits in original number should be the total number of digits AFTER the decimal point for the final answer for antilog = the total number of digits after the decimal point appearing in the exponent will be the total number of digits in the final answer Ex: log 23.5 = 1.371 ~ or ~ 101.371 = 23.5 (a) if we begin with an initial SO2Cl2 pressure of 340 torr, what is the pressure of this substance after 75s? (b) At what time will the pressure of SO2Cl2 decline to one tenth its initial value? 4. The first-order rate constant for the decomposition of N2O5. N2O5 (g) → 2 NO2 (g) + O2 (g) At 70oC is 6.82 x 10 – 3 s – 1. Suppose we start with 0.0250 mol of N2O5 (g) in a volume of 2.0 L. (a) How many moles of N2O5 will remain after 2.5 min? Follow ALL math work rules! (b) How many minutes will it take for the quantity of N2O5 to drop to 0.010 mol? Follow ALL math work rules! 2 4. Continued: (c) What is the half-life of N2O5 at 70oC? Follow ALL math work rules! 5. From the following data for the first-order gas-phase isomerization of CH3NC at 215oC, calculate the firstorder rate constant and half-life for the reaction: Pressure Time(s) CH3NC(torr) 0 2,000 5,000 8,000 12,000 15,000 ln PCH3NC 502 335 180 95.5 41.7 22.4 ln P vs t 7 ln P (CH3NC) 6 5 4 3 2 1 0 0 2 4 6 8 10 12 14 16 Time (sec) x 103 3 6. Consider the data presented in Problem 2 of the 14.1, 14.2 Homework. (a) Determine whether the reaction is a first-order or second-order reaction. Time (s) [A] ln[A] 1/ [A] Graph 1: ln[A] vs time Time (s) 0 40 80 120 160 -2.30 -2.50 -2.70 ln [A] -2.90 -3.10 -3.30 -3.50 -3.70 -3.90 Graph 2: 𝟏 [𝑨] vs time 60 50 1/ [A] 40 30 20 10 0 0 40 Time (s) 80 120 160 4 6. Continued: (b) What is the value of the rate constant? (c) What is the half-life? 7. Sucrose, C12H22O11, which is commonly known as table sugar, reacts in dilute acid solutions to form two simpler sugars, glucose and fructose, both of which have the formula C6H12O6: C12H22O11(aq) + H2O(l) → 2C6H12O6(aq) At 23oC and in 0.5M HCl, the following data were obtained for the disappearance of sucrose: Time (min) [C12H22O11] (M) 0 39 80 140 210 0.316 0.274 0.238 0.190 0.146 (a) Is the reaction first order or second order with respect to [C12H22O11]? Explain Graph 1: ln[A] vs time -0.90 -1.00 ln [C11H22O11] -1.10 -1.20 -1.30 -1.40 -1.50 -1.60 -1.70 -1.80 -1.90 -2.00 0 10 20 30 40 50 60 70 80 90 100 110 120 130 140 150 160 170 180 190 200 210 220 Time (min) 5 7. Continued: Graph 2: 𝟏 [𝑨] vs time 7.00 6.50 1/ [C11H22O11] 6.00 5.50 5.00 4.50 4.00 3.50 3.00 2.50 2.00 1.50 0 10 20 30 40 50 60 70 80 90 100 110 120 130 140 150 160 170 180 190 200 210 220 Time (min) (b) What is the value of the rate constant? 6