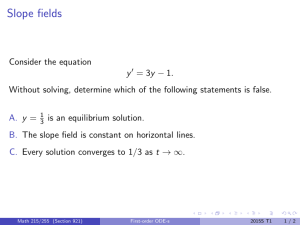
Text Questions
advertisement

14TQ: Chemical Kinetics Name: _________________________ Text Questions from Brown, et. al. 1. What is chemical kinetics? 14.1 2. Why do the speeds of reactions depend on the nature of the reactants themselves? 3. Why do reactions involving solids proceed faster if the surface area of the solid is increased? 4. Why does reaction rate increase with increasing concentration? 5. Why do rates increase with increasing temperature? 6. What are catalysts? 7. What two conditions must a collision meet in order for it to lead to a reaction? 14.2 8. Define “reaction rate” and give its typical units. 9. What is “” equal to? 10. Rates of appearance are written with __ signs, while rates of disappearance are written with __ signs. 11. Why is it typical for rates to decrease as a reaction proceeds? 12. A decreasing rate of reaction is shown on a “concentration v. time” graph by what? 13. What is an instantaneous rate? 14. How do we define the initial rate of a reaction? 14.3 15. What is a rate law? 16. The rate constant k changes with… 17. We can calculate the value of the rate constant k if we know what two things? 18. Write the general form of the rate law for most reactions. 19. What are the exponents n and m called? 20. What is the overall reaction order? 21. How must the reaction orders (i.e., the exponents in a rate law) be determined? 22. The rate law for any chemical reaction cannot be predicted merely by… 23. If a reaction order is _A, B, C_ in a particular reactant, then changing its concentration will produce _A, B, C_ changes in the reaction rate. A. 0 _____________ B. 1 _____________ C. 2 _____________ 24. What does the rate constant NOT depend on? What two things DO affect the rate constant? 14.4 25. What is a first-order reaction? 26. Write the two forms of the integrated rate law for a first-order reaction. 27. What three things can you use the equations from Q26 to determine? 28. For a first-order reaction, a graph of ln[A]t versus time gives a straight line with a slope of ___ and a y-intercept of _____. 29. Why can we use pressure as a unit of concentration for a gas? 30. A second-order reaction is one whose rate depends on one of what two things? 31. Write the integrated rate law for a second-order reaction that is second order in a single reactant. 32. For a second-order reaction, a graph of 1/[A]t versus time gives a straight line with a slope of __ and a y-intercept of _____. 33. What is one way to distinguish between first- and second-order rate laws? 34. What is the half-life t1/2 of a reaction? 35. For a first-order reaction, the half-life remains ___________ throughout the reaction and does NOT depend on the ___________ ________________. 36. What other process – besides some chemical reactions – is a first-order process? 37. Unlike for first-order reactions, the half-life for second-order reactions depends on ________________. 14.5 38. For most reactions, what is the relationship between temperature and reaction rate? 39. Faster rates at higher temperatures are due to what? 40. What is the central idea of the collision model? 41. What determines whether the atoms are suitably positioned to form new bonds? 42. Upon collision, the kinetic energy of the molecules can be used to do what three things to bonds? 43. What must colliding molecules have in order to react? 44. What is the activated complex, and what is another name for it? 45. What effect does the total energy change E have on the reaction rate? 46. What is the relationship between the magnitude of the activation energy and the reaction rate? 47. With regard to activation energy, why is there a greater reaction rate at a higher temperature? 48. By graphing ln k versus 1/T, one obtains a line having a slope of… 14.6 49. What does a balanced equation tell us? What does it NOT tell us? 50. Single events (i.e., single steps in a reaction process) are called… 51. What is molecularity? Also, list the names of three types of molecularity. 52. A sequence of elementary reactions is a __________ ____________. Such a process always involves _________________, which are produced in one elementary reaction and consumed in another. 53. A. Write the rate law for a unimolecular process. B. Write the rate law for a bimolecular process. 54. The rate law for any elementary reaction follows directly from the _______________ of that reaction; however, we cannot say anything about a mechanism from the overall __________ _________ alone. 55. Give two names for the slowest elementary step in a reaction mechanism. 56. In a two-step mechanism, when the first step is slow, the rate law of the overall reaction equals… 57. In a two-step mechanism, when the first step is fast, you need to solve for the concentration of an intermediate by… 14.7 58. What is a homogeneous catalyst? 59. A heterogeneous catalyst is usually a _______ in contact with… 60. What is the difference between adsorption and absorption? 61. What ARE enzymes and what mass do they typically have? 62. In an enzyme, what is the relationship between the active site and the substrate?