review for the final
advertisement

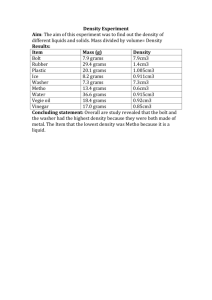
Review: 1. a. b. c. d. e. f. g. h. i. j. 2. a. b. c. d. e. f. g. h. Definitions Define and give an example of : Homogeneous mixture Heterogeneous mixture Ionic compound Covalent bonding What is the difference between an electrolyte and a non-electrolyte? Give two examples of each. Enthalpy of formation Theoretical yield Ionization energy Electronegativity Molar volume of a gas Chemical equations: Reaction of sodium with water. One of the products is hydrogen gas. Write a balanced molecular equation. Combustion of propane. Write a balanced molecular equation. The precipitation of lead chloride from lead (II) nitrate and sodium chloride. Write a balanced molecular equation. The oxidation of iron metal by silver nitrate. Assume the +2 ion forms. Write a net ionic equation. The decomposition of hydrogen peroxide into water and oxygen. Write the balanced molecular equation. Neutralization of sulfuric acid by sodium hydroxide. Write a balanced molecular equation. The oxidation of calcium by a strong acid. Write a net ionic equation. The precipitation of lead (II) iodide from potassium iodide and lead (II) nitrate. Problems: 3. Density a. Lead has a density of 11.34 g/ml. Find the volume in cubic centimeters of a piece of lead that weighs 5.00 pounds. (2.2 lbs = 1 kg) b. Water has a density of 1.00g/ml. What is the mass of a cubic meter of water in kg? in lbs? 4. Stoichiometry: A. a. Glucose is used as a source of energy for the human body. The overall reaction is C6H12O6 + 6O2 → 6 CO2 + 6 H2O Glucose + oxygen → carbon dioxide + water a. Calculate the number of grams water produced when 21.5 grams of glucose reacts completely with excess oxygen. b. Calculate the number of grams of glucose that would be required to react completely with 5.35 liters of oxygen at STP. c. Calculate the mass of carbon dioxide produced when 20.0 grams of glucose reacts completely with excess oxygen. Calculate the volume of this gas at 40.0 C and .987 atm. B. Ammonia is synthesized from hydrogen and nitrogen according to the following reaction. N2 + 3H2 → 2 NH3 a. If excess hydrogen is reacted with 110.0 grams of nitrogen, how many grams of ammonia would be produced? b. What volume would this ammonia gas occupy at STP? C. Given the reaction: 2Al + 3H2SO4→ 3H2 + Al2(SO4)3 a. How many grams of aluminum sulfate would be produced when 55.2 grams of aluminum reacts completely with excess sulfuric acid? b. How many liters of hydrogen gas at STP would be produced when 3.00 moles of sulfuric acid reacts completely with excess aluminum? 5. A gas has a volume of 250 ml at 70 ⁰C. What volume does it occupy at _40 ⁰C? 6. Calculate the pressure exerted by 0.375 moles of oxygen gas in a 250 ml flask at 40⁰C. 7. Use table 8.4 in your text (p.326) to estimate the bond enthalpy for the reaction # 8.65 a on page 336. 8. An atom absorbs a photon of wavelength 350 nm. Calculate the change in energy of the atom in Joules. 9. 47.5 ml of 0.25 M sodium hydroxide is used to neutralize 25.00 ml of sulfuric acid. a. Write the balanced equation for this reaction. b. Calculate the concentration of the sulfuric acid.