Vocab Nuclear
advertisement

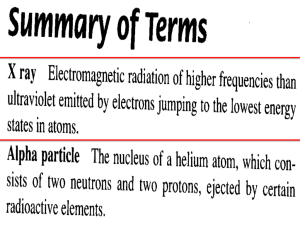
Vocab Unit D: Term Proton Definition Charge: Mass; Location: Electron Charge: Mass; Location: Neutron Charge: Mass; Location: Isotope Radioactivity Radioisotope Transmutation Alpha Particle Alpha Decay With example Charge: Penetrating Power: Symbol: Mass; Beta Particle Charge: Mass; Penetrating Power: Symbol: Beta Decay With example Gamma Ray Charge: Mass; Penetrating Power: Symbol: Positron Charge: Penetrating Power: Symbol: Positron Emission With example Mass Number Nuclear Fission Nuclear Fusion Emit (Emission) Nuclear Charge Half-Life Mass; Tracer Decay Decay mode Atomic number MATCHING: after completing the vocab definitions on the previous pages, try to match the correct vocab term with my definition; some of your words may be used more than once _________________________positive subatomic particle in the nucleus _________________________ invisible rays emitted by radioactive substances ________________________negative subatomic particle outside the nucleus ________________________identifies an atom; equal to the number of protons ________________________particle in the nucleus with no charge ________________________to break down ________________________includes the mass of protons and neutrons in a single atom ________________________ shows types of particles emitted by radioisotope (mode) ________________________subatomic particle that has the opposite charge to a proton, found outside the nucleus ________________________isotope of an atom that is radioactive ________________________equal to the charge of all the protons in the nucleus ________________________radioactive isotope used to trace or follow a reaction ________________________the amount of time it takes for one half of a radioactive isotope to decay or transmutate into another element ________________________to give off or release ________________________when a large nucleus is split, releases huge amount of energy ________________________ high energy electron that leaves the nucleus ________________________when small nuclei like H and He come together, releases huge amount of energy ________________________particle with mass of electron but positive charge ________________________ particle with a mass of 4 and charge of +2 ________________________ has no mass and no charge ________________________subatomic particle with the same mass as a proton ________________________change of one element into another element ________________________ emitted when proton changes into a neutron ________________________elements with the same atomic number but a different mass number ________________________emitted when a neutron changes into a proton _______________________subatomic particle with a negligible mass ________________________atoms of the same element with a different number of neutrons _______________________ ________________________ ________________________ ________________________