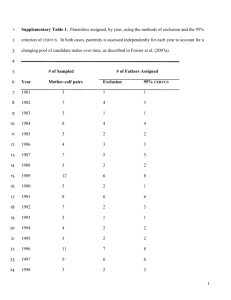
mec12934-sup-0007-DataS1
advertisement

Supporting information about data This document includes three sections (1) Data bibliography (2) Details of data extraction and effect size averaging, and (3) List of relevant papers excluded from the present study. Data bibliography: The references below are those from which data were extracted to calculate effect sizes for metaanalyses. Actual effect sizes and associated information, such as moderator variables and species names can be found in the supporting electronic data files. Aeschlimann PB, Häberli MA, Reusch TBH, Boehm T, Milinski M (2003) Female sticklebacks Gasterosteus aculeatus use self-reference to optimize MHC allele number during mate selection. Behavioral Ecology and Sociobiology, 54, 119–126. Agbali M, Reichard M, Bryjová A, Bryja J, Smith C (2010) Mate choice for nonadditive genetic benefits correlate with MHC dissimilarity in the rose bitterling (Rhodeus ocellatus). Evolution, 64, 1683–1696. Bahr A, Sommer S, Mattle B, Wilson AB (2012) Mutual mate choice in the potbellied seahorse (Hippocampus abdominalis). Behavioral Ecology, 23, 869–878. Bonneaud C, Chastel O, Federici P, Westerdahl H, Sorci G (2006) Complex MHC-based mate choice in a wild passerine. Proceedings of the Royal Society B: Biological Sciences, 273, 1111–1116. Bos DH, Williams RN, Gopurenko D, Bulut Z, Dewoody JA (2009) Condition-dependent mate choice and a reproductive disadvantage for MHC-divergent male tiger salamanders. Molecular Ecology, 18, 3307–3315. Cutrera AP, Fanjul MS, Zenuto RR (2012) Females prefer good genes: MHC-associated mate choice in wild and captive tuco-tucos. Animal Behaviour, 83, 847–856. Egid K, Brown JL (1989) The major histocompatibility complex and female mating preferences in mice. Animal Behaviour, 38, 548–549. Ehman KD, Scott ME (2001) Urinary odour preferences of MHC congenic female mice, Mus domesticus: implications for kin recognition and detection of parasitized males. Animal Behaviour, 62, 781–789. Eizaguirre C, Yeates SE, Lenz TL, Kalbe M, Milinski M (2009) MHC-based mate choice combines good genes and maintenance of MHC polymorphism. Molecular Ecology, 18, 3316–3329. Ekblom R, Sæther SA, Grahn M et al. (2004) Major histocompatibility complex variation and mate choice in a lekking bird, the great snipe (Gallinago media). Molecular Ecology, 13, 3821–3828. Eklund A, Egid K, Brown JL (1991) The major histocompatibility complex and mating preferences of male mice. Animal Behaviour, 42, 693–694. Evans ML, Dionne M, Miller KM, Bernatchez L (2012a) Mate choice for major histocompatibility complex genetic divergence as a bet-hedging strategy in the Atlantic salmon (Salmo salar). Proceedings of the Royal Society B: Biological Sciences, 279, 379–386. Evans ML, Neff BD, Heath DD (2012b) Behavioural and genetic analyses of mate choice and reproductive success in two Chinook salmon populations. Canadian Journal of Fisheries and Aquatic Sciences, 70, 263–270. Freeman-Gallant CR, Meguerdichian M, Wheelwright NT, Sollecito SV (2003) Social pairing and female mating fidelity predicted by restriction fragment length polymorphism similarity at the major histocompatibility complex in a songbird. Molecular Ecology, 12, 3077–3083. Gillingham MAF, Richardson DS, Løvlie H et al. (2009) Cryptic preference for MHC-dissimilar females in male red junglefowl, Gallus gallus. Proceedings of the Royal Society B: Biological Sciences, 276, 1083–1092. Griggio M, Biard C, Penn DJ, Hoi H (2011) Female house sparrows “count on” male genes: experimental evidence for MHC-dependent mate preference in birds. BMC Evolutionary Biology, 11, 44. Huchard E, Baniel A, Schliehe-Diecks S, Kappeler PM (2013) MHC-disassortative mate choice and inbreeding avoidance in a solitary primate. Molecular Ecology, 22, 4071–4086. Huchard E, Knapp LA, Wang J, Raymond M, Cowlishaw G (2010) MHC, mate choice and heterozygote advantage in a wild social primate. Molecular Ecology, 19, 2545–2561. Jäger I, Eizaguirre C, Griffiths SW et al. (2007) Individual MHC class I and MHC class IIB diversities are associated with male and female reproductive traits in the three-spined stickleback. Journal of Evolutionary Biology, 20, 2005–2015. Kalbe M, Eizaguirre C, Dankert I et al. (2009) Lifetime reproductive success is maximized with optimal major histocompatibility complex diversity. Proceedings of the Royal Society B: Biological Sciences, 276, 925–934. Knafler GJ, Clark JA, Boersma PD, Bouzat JL (2012) MHC diversity and mate choice in the magellanic penguin, Spheniscus magellanicus. Journal of Heredity, 103, 759–768. Landry C, Garant D, Duchesne P, Bernatchez L (2001) “Good genes as heterozygosity”: the major histocompatibility complex and mate choice in Atlantic salmon (Salmo salar). Proceedings of the Royal Society B: Biological Sciences, 268, 1279–1285. Løvlie H, Gillingham MAF, Worley K, Pizzari T, Richardson DS (2013) Cryptic female choice favours sperm from major histocompatibility complex-dissimilar males. Proceedings of the Royal Society B: Biological Sciences, 280, 20131296. McCairns RJS, Bourget S, Bernatchez L (2011) Putative causes and consequences of MHC variation within and between locally adapted stickleback demes. Molecular Ecology, 20, 486–502. Miller HC, Moore JA, Nelson NJ, Daugherty CH (2009) Influence of major histocompatibility complex genotype on mating success in a free-ranging reptile population. Proceedings of the Royal Society B: Biological Sciences, 276, 1695–1704. Nelson NJ, Daugherty CH (2009) Influence of major histocompatibility complex genotype on mating success in a free-ranging reptile population. Proceedings of the Royal Society B: Biological Sciences, 276, 1695–1704. Olsson M, Madsen T, Nordby J et al. (2003) Major histocompatibility complex and mate choice in sand lizards. Proceedings of the Royal Society B: Biological Sciences, 270, S254–S256. Olsson M, Madsen T, Ujvari B, Wapstra E (2004) Fecundity and MHC affects ejaculation tactics and paternity bias in sand lizards. Evolution, 58, 906–909. Paterson S, Pemberton JM (1997) No evidence for major histocompatibility complex-dependent mating patterns in a free-living ruminant population. Proceedings of the Royal Society B: Biological Sciences, 264, 1813–1819. Penn D, Potts W (1998) MHC-disassortative mating preferences reversed by cross-fostering. Proceedings of the Royal Society B: Biological Sciences, 265, 1299–1306. Potts WK, Manning CJ, Wakeland EK (1991) Mating patterns in seminatural populations of mice influenced by MHC genotype. Nature, 352, 619–621. Radwan J, Tkacz A, Kloch A (2008) MHC and preferences for male odour in the bank vole. Ethology, 114, 827–833. Reusch TB, Häberli MA, Aeschlimann PB, Milinski M (2001) Female sticklebacks count alleles in a strategy of sexual selection explaining MHC polymorphism. Nature, 414, 300–302. Richardson DS, Komdeur J, Burke T, Schantz T von (2005) MHC-based patterns of social and extra-pair mate choice in the Seychelles warbler. Proceedings of the Royal Society B: Biological Sciences, 272, 759–767. Roberts SC, Gosling LM (2003) Genetic similarity and quality interact in mate choice decisions by female mice. Nature Genetics, 35, 103–106. Sauermann U, Nürnberg P, Bercovitch F et al. (2001) Increased reproductive success of MHC class II heterozygous males among free-ranging rhesus macaques. Human Genetics, 108, 249–254. Schwensow N, Fietz J, Dausmann K, Sommer S (2008) MHC-associated mating strategies and the importance of overall genetic diversity in an obligate pair-living primate. Evolutionary Ecology, 22, 617– 636. Setchell JM, Charpentier MJE, Abbott KM, Wickings EJ, Knapp LA (2010) Opposites attract: MHCassociated mate choice in a polygynous primate. Journal of Evolutionary Biology, 23, 136–148. Sherborne AL, Thom MD, Paterson S et al. (2007) The genetic basis of inbreeding avoidance in house mice. Current Biology, 17, 2061–2066. Skarstein F, Folstad I, Liljedal S, Grahn M (2005) MHC and fertilization success in the Arctic charr (Salvelinus alpinus). Behavioral Ecology and Sociobiology, 57, 374–380. Sommer S (2005) Major histocompatibility complex and mate choice in a monogamous rodent. Behavioral Ecology and Sociobiology, 58, 181–189. Strandh M, Westerdahl H, Pontarp M et al. (2012) Major histocompatibility complex class II compatibility, but not class I, predicts mate choice in a bird with highly developed olfaction. Proceedings of the Royal Society B: Biological Sciences, 279, 4457–4463. Thom MD, Stockley P, Jury F et al. (2008) The direct assessment of genetic heterozygosity through scent in the mouse. Current Biology, 18, 619–623. Wedekind C, Chapuisat M, Macas E, Rülicke T (1996) Non-random fertilization in mice correlates with the MHC and something else. Heredity, 77 ( Pt 4), 400–409. Westerdahl H (2004) No evidence of an MHC-based female mating preference in great reed warblers. Molecular Ecology, 13, 2465–2470. Yamazaki K, Boyse EA, Miké V et al. (1976) Control of mating preferences in mice by genes in the major histocompatibility complex. Journal of Experimental Medicine, 144, 1324–1335. Yamazaki K, Yamaguchi M, Andrews PW, Peake B, Boyse EA (1978) Mating preferences of F2 segregants of crosses between MHC-congenic mouse strains. Immunogenetics, 6, 253–259. Yeates SE, Einum S, Fleming IA et al. (2009) Atlantic salmon eggs favour sperm in competition that have similar major histocompatibility alleles. Proceedings of the Royal Society B: Biological Sciences, 276, 559–566. Details of data extraction and effect size averaging Details of data extraction from papers where test statistics other than the standard effect size were reported, or from which we calculated the average effect size from multiple test statistics are listed below: Publication Details Agbali et al. 2010 The mean effect size was calculated among different measures of dissimilarity (i.e. dissimilarity based on 1. functional distance in amino acid sequence, 2. phylogenetic distance in amino acid sequence, 3. strongly positively selected sites, and 4. positively selected sites). Bahr et al. 2012 The weighted mean effect size was calculated among different measures of mating preference (i.e. preference based on 1. olfactory cues only and 2. visual and olfactory cues). Bonneaud et al. 2006 The mean effect size for similarity was calculated between mated vs. random and mated vs non-mated. Cutrera et al. 2012 The weighted (for sample size) mean effect size among olfactorybased preference, confined male preference and tethered male preference was calculated. The weighted mean effect size was then calculated between wild and captive populations. Ehman & Scott 2001 The proportion of time spent with MHC similar males was compared against the null expectation of 50% and an effect size was computed using the effect size calculator for proportional data. Eklund 1990 The number of males that mated with the identical-MHC females was analysed using Chi-squared test for goodness of fit. The weighted (for sample size) mean effect size between GAA and CHR haplotypes was then calculated. Evans et al. 2013 The effect size, r, was calculated from the p-value. The sample size, and hence df for this calculation was based on the number of chosen sex individuals (as opposed to the number of choosy sex individuals) since the permutation tests were carried out based on the number of chosen sex individuals. The weighted mean effect sizes were calculated between the populations, Little Qualicum and Quinsam. Freeman-Gallant et al. The number of females that mated with MHC similar males was 2003 analysed using Chi-squared test for goodness of fit. The effect size was then calculated from the Chi-squared values. The mean effect size was calculated between the two age groups, i.e. yearlings and old females. Gillingham et al. 2009 The mean effect sizes were calculated among different similarity measures (i.e. number of alleles and number of haplotypes shared) and simultaneous and sequential choice for both pre- and post-copulatory choices. Griggio et al. 2011 The proportion of time spent by focal female (i.e. a measure of preference) was extracted from Figure 2 using GetData (www.getdata-graph-digitizer.com). The mean effect size among Low-High, Low-Intermediate, Intermediate-High males was then calculated for each class (i.e. low, intermediate, and high) of females. Then the average effect size was calculated among the three classes of females. Huchard et al. 2010 The mean effect sizes were calculated among different similarity measures (i.e. number of sequence, supertype & haplotype). The results for female reproductive success were left out as the information on the choosing males was unavailable (hence it was decided that the female reproductive success was not presented as a proxy of male choice). Data for all females were used. Three average effect sizes were calculated from this paper. 1) The average effect size between MHC loci for band-based dissimilarity measures; 2) The average effect size calculated between different MHC loci (i.e. DRB & DQB) and dissimilarity measures for sequence-based data (i.e. mean number of amino acid differences between the 4 possible combinations of a pair, number of amino acid differences between the most similar haplotypes (sequences) of a pair), mean functional distance between the 4 possible haplotypes (sequences) combinations of a pair, functional distance between the most similar haplotypes (sequences) of a pair; 3) The average effect size between different MHC loci (i.e. DRB & DQB) and diversity measures (i.e. number of amino acids that differ between the 2 haplotypes (sequences) possessed by the mate and functional distance between the 2 haplotypes (sequences) possessed by the mate). The results partially overlapping with Evans et al. 2012 Proc B were not included in the present analysis. The average effect sizes were calculated between major and minor loci within MHC classes. Additionally for female choice, the effect sizes were further averaged between two measures of cryptic preference (i.e. sperm number on eggs and ejaculate ejection) The total number of combined, unique PBR sequences per potential couple was not included as it is a measure of both diversity and dissimilarity of the chosen mate. The weighted average between the demes and environments were calculated. The weighted (for sample size) mean effect size between the three different measures of preference (i.e. visit to side, time spent and number of visits in box) was calculated. From Table 2b, only the expressed MHC Class 2 marker (i.e. OLADRB) and the Class 1 marker (i.e. OMHC1) were included, Huchard et al. 2013 Landry et al. 2001 Løvlie et al. 2013 McCairns et al. 2011 Olsson et al. 2003 Paterson & Pemberton 1997 Penn & Potts 1998 Potts et al. 1991 Sauermann et al. 2001 Setchell et al. 2010 Sherborne et al. 2007 Schwensow et al. 2008 Strandh et al. 2012 since other loci were either non-expressed or flanking marker controls. For each class, a Chi-squared test (comparing sharing 0 to 1 and 2) was conducted and the average Chi-squared value was calculated, from which the effect size, r, was calculated. The results from the out-fostering portion were excluded as postbirth conditioning was unique to this paper and also not the focus of our study. The mating score was analysed using Chi-squared test for goodness of fit. Also the number of females that mated with MHC similar males was analysed using Chi-squared test for goodness of fit. The effect size was then calculated from the Chisquared values. From Table 1, a Chi-squared value was calculated from the sum of observed and expected MHC heterozygosity / homozygosity of 9 populations. The sample size was approximated as the sum of males involved. From Table 2, Chi-squared values were calculated from the observed and expected MHC heterozygosity / homozygosity of nestlings and embryos. Additionally, a Chisquared value was calculated from the average observed and expected MHC heterozygosity / homozygosity of informative laboratory matings. From Table 3, Chi-squared values were calculated from the observed and expected MHC heterozygosity / homozygosity of territorial and extra-territorial matings; corresponding sample sizes were taken from the text. Then the weighted average effect size was calculated between the number of nestlings and embryos and between laboratory matings and territorial matings. The weighted mean effect size was calculated between wild and captive populations. The average effect size was calculated between band-based dissimilarity measures (i.e. number of sequences and supertypes difference). Likewise for diversity measures, the average effect size was calculated between the number of sequences and number of supertypes. From Table S2, a Chi-squared test (comparing sharing 0 to 1 and 2) was conducted for each of the MHC related models (i.e. number of matings, number of offspring, and offspring MHC genotypes). Then the weighted average effect size was calculated for the measures of reproductive success (i.e. the number of matings and offspring). The weighted average effect sizes were calculated between MHC measures based on alleles and supertypes, between social fathers and genetic fathers, and between extra-pair mating comparisons based on ‘cuckolded social males vs non-cuckolded males’ and ‘cuckolded social males vs genetic EPY fathers’ The average effect sizes between phylogenetic and functional Thom et al. 2008 Wedekind et al. 1996 Westerdahl 2004 Yamazaki et al. 1976 Yamazaki et al. 1978 diversity were calculated. The weighted (for sample size) mean effect size between different measures of preference (territory, nest and nest + male) was calculated. Only the overall proportion of homozygosity was used as the main findings of the paper (i.e. the change in heterozygosity over time was out of the scope of the present analysis). The mean effect size for similarity was calculated between mated vs. random and mated vs average. Since it was unclear whether results were derived from single or multiple experiments, only the results shown in Figure 1 were extracted for each cross separately (results reported in Table 2 and 3 are likely to be from the same experiment). The weighted average proportion for each cross was calculated based on the sample size and the proportion of males that preferred MHC dissimilar females was analysed using Chi-squared test for goodness of fit. The effect size was then calculated from the Chisquared values. Then the weighted average effect size among the three crosses was calculated. The proportion of preference for dissimilar mates was extracted using GetData (www.getdata-graph-digitizer.com).The weighted (for sample size) average dissimilarity preference for the BALB/BALB.B strain was calculated for Figure 2 Group 2, 5, and 6 and Figure 3 Group 2-4. Group 3 and 4 from Figure 2 were excluded because the equivalent comparisons are not found in Figure 3. Similarly, the weighted (for sample size) average preference for B6/B6.H-2k strain was calculated for Group 2 from Figure 4. In both cases Group 1 was excluded because the data come from a previously published paper (i.e. Yamazaki et al. 1976 J Exp Med). Then the weighted average effect size among crosses and strains was calculated. List of excluded papers Relevant studies excluded from the presented study and the reason for exclusion are listed below Publication Reasons Beauchamp et al. 2000 Female genotypes are identical between treatments. Beauchamp et al. 1988 Table 1 & 2, Figure 2 & 3 are from Yamazaki et al. (1976) & (1978). In Figure 4, the genetic identity of choosy females is unclear. Consuegra & de Leaniz 2008 Only offspring MHC dissimilarity was reported. Garner et al. 2010 Only offspring MHC dissimilarity was reported. Wedekind et al. 2004 Test statistics for the association between MHC genotypes and fertilisation are reported while no information with regard to diversity, heterozygosity or dissimilarity is available. Neff et al. 2008 Only offspring MHC dissimilarity was reported. Schwensow et al. 2008 Some effect sizes (for male choice) were reported without slopes, but the same data were used by Huchard et al. (2013) Beauchamp GK, Curran M, Yamazaki K (2000) MHC-mediated fetal odourtypes expressed by pregnant females influence male associative behaviour. Animal Behaviour, 60, 289–295. Beauchamp GK, Yamazaki K, Bard J, Boyse EA (1988) Preweaning experience in the control of mating preferences by genes in the major histocompatibility complex of the mouse. Behavior Genetics, 18, 537– 547. Consuegra S, Leaniz CG de (2008) MHC-mediated mate choice increases parasite resistance in salmon. Proceedings of the Royal Society B: Biological Sciences, 275, 1397–1403. Garner SR, Bortoluzzi RN, Heath DD, Neff BD (2010) Sexual conflict inhibits female mate choice for major histocompatibility complex dissimilarity in Chinook salmon. Proceedings of the Royal Society B: Biological Sciences, 277, 885–894. Neff BD, Garner SR, Heath JW, Heath DD (2008) The MHC and non-random mating in a captive population of Chinook salmon. Heredity, 101, 175–185. Schwensow N, Eberle M, Sommer S (2008) Compatibility counts: MHC-associated mate choice in a wild promiscuous primate. Proceedings of the Royal Society B: Biological Sciences, 275, 555–564. Wedekind C, Walker M, Portmann J et al. (2004) MHC-linked susceptibility to a bacterial infection, but no MHC-linked cryptic female choice in whitefish. Journal of Evolutionary Biology, 17, 11–18.