Experiment One - Calibration and Choosing Glassware
advertisement

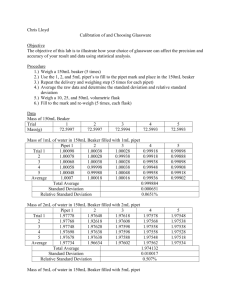
Orlando 1 Quantitative Analytical Chemistry Joseph Orlando Lab #1: Calibration and Choosing Glassware Purpose: The objective of this experiment is to calibrate several pieces of volumetric glassware; 1, 2 and 5 mL pipets as well as 10, 25 and 50 mL volumetric flasks. Statistical analysis will be performed to determine the accuracy and precision of the measurements taken and to also decide which pipet and volumetric flask are the most precise/accurate. Procedure: Calibration of Pipets A clean, dry 100mL beaker was weighed five times and the mean mass was calculated. Water was delivered to the 100mL beaker (following proper pipetting techniques which were discussed in lab) with 1, 2 and 5mL pipettes filled to the appropriate line. This was repeated five times for each pipette. Mass of the beaker and water delivered via the pipette was recorded and averaged for each pipette. Standard deviation was also calculated. Calibration of Volumetric Glassware 10, 25 and 50mL volumetric flasks were cleaned, dried and then weighed five times while empty. The empty mass was recorded and averaged for each volumetric flask. The flasks were filled to the appropriate line and mass was recorded. This was repeated five times for each flask and the mass of the flask plus water was recorded and averaged for each size flask. Standard deviation was calculated. Data: Calibration of Pipets Trial # Mass (g) of Empty 100 mL Beaker 1 51.0198 2 51.0202 3 51.0201 4 51.0191 5 51.0191 Average Mass 51.0197 Standard Deviation 0.0004 Orlando 2 Trial # Mass (g) of Flask using 1-mL Pipet Mass (g) of Water 1 52.0130 0.9938 2 51.9877 0.9680 3 52.0132 0.9935 4 52.0053 0.9856 5 52.0193 0.9996 Avg. Mass: 0.988 Standard Deviation 0.0109 Relative Standard Deviation 1.1032% Using 2-mL Pipet Mass (g) of Water Using 5-mL Pipet Mass (g) of Water 53.0139 1.9942 55.9878 4.9681 53.0163 1.9966 55.9831 4.9634 53.0021 1.9824 55.9762 4.9565 53.0224 2.0027 55.9720 4.9523 53.0047 1.9850 55.9868 4.9671 Avg. Mass: 1.9921 Standard Deviation Relative Standard Deviation 4.9614 0.0075 0.0061 0.3764% 0.1229% Calibration of Volumetric Glassware Avg. Mass (g) of Empty 10 mL Flask 13.1074 Trial # Mass (g) 10-mL Flask w/ Water Mass (g) of Water 1 23.0726 9.9646 2 23.0582 9.9512 3 23.0845 9.9769 4 23.0766 9.9695 5 23.1113 10.0041 Avg. Mass: 9.9732 Standard Deviation 0.0175 Relative Standard Deviation 0.1754% Orlando 3 Avg. Mass (g) of Empty 25 mL Flask Avg. Mass (g) of Empty 50 mL Flask 17.9918 48.3933 Mass (g) 25-mL Flask w/ Water Mass (g) Water Mass (g) 50-mL Flask w/ Water Mass (g) Water 42.8726 24.8799 98.0925 49.6985 42.9024 24.9107 98.2089 49.8159 42.9118 24.9199 98.1884 49.7951 42.9102 24.9190 98.1996 49.8062 42.8527 24.8612 98.1936 49.8008 Avg. Mass: 24.8981 49.7833 Standard Deviation 0.0235 0.0429 0.0943% 0.0861% Relative Standard Deviation Equations: 𝑀𝑒𝑎𝑛 = ∑𝑖 𝑥𝑖 𝑛 𝑒𝑥𝑎𝑚𝑝𝑙𝑒: (13.1080 + 13.1070 + 13.1076 + 13.1071 + 13.1072) = 13.1074 5 𝑆𝑡𝑎𝑛𝑑𝑎𝑟𝑑 𝐷𝑒𝑣𝑖𝑎𝑡𝑖𝑜𝑛 ∑𝑖(𝑥𝑖 − 𝑥̅ )2 = √ 𝑛−1 (0.9938 − 0.9880) + (0.9680 − 0.9880) + (0.9935 − 0.9880) + (0.9856 − 0.9880) + (0.9996 − 0.9880)2 𝑒𝑥𝑎𝑚𝑝𝑙𝑒: √ 4 𝑅𝑒𝑙𝑎𝑡𝑖𝑣𝑒 𝑠𝑡𝑎𝑛𝑑𝑎𝑟𝑑 𝑑𝑒𝑣𝑖𝑎𝑡𝑖𝑜𝑛 = 100 𝑥 𝑠 𝑥̅ 𝑒𝑥𝑎𝑚𝑝𝑙𝑒: 100 𝑥 0.0175 = 0.1754 9.9732 Conclusion: Based on the results of this experiment, it was determined that the 5mL pipet and the 10mL volumetric flask were the most precise pieces of glassware. This determine was based off of the standard deviations that were calculated for the different sized glassware. The 5mL pipet and the 10mL volumetric flask had the smallest standard deviations. The volumetric flasks were more accurate than the pipets overall. Human error, such as the measurement of the water, should be considered when observing the calculated deviations from this experiment. Orlando 4 Post Lab Questions: 1. Explain using calculations and words whether it is better to use 20, 49, or 56 mL of solution from a 50 mL buret. It is best to use 49mL of solution when using a 50mL buret. If we were to use 56mL of solution we would have to fill the buret up twice which would increase the chance for error in our measurements. Also if were to only use 20mL of solution this would decrease the precision of the measurement. As the data from the experiment shows, increasing the volume increases the precision of the measurement. 2. Assuming the volume of base used had a mean value of 43.56 mL with a standard deviation of 0.89 mL using five titrations, the molarity of base was 0.1012 M standard deviation 0.0025, and the volume of acid had a mean value of 50 mL standard deviation 0.05 mL, what would be the calculated molarity and error associated with this measurement? 𝑉𝑏𝑎𝑠𝑒 × 𝑀𝑏𝑎𝑠𝑒 = 𝑉𝑎𝑐𝑖𝑑 × 𝑀𝑎𝑐𝑖𝑑 (. 04356 𝐿) × (. 1012 𝑀) = (.050 𝐿) × 𝑀𝑎𝑐𝑖𝑑 𝑀𝑎𝑐𝑖𝑑 = 0.0882 𝑀 Propagation of Error: .89 𝑚𝐿 2 ) 43.56 𝑚𝐿 % 𝐸𝑟𝑟𝑜𝑟 = √( .0025 𝑚𝐿 2 ) .1012 𝑚𝐿 +( .05 𝑚𝐿 2 ) 50 𝑚𝐿 +( = 3.21% Macid = 0.0882 M with 3.21% error, or 0.0882 ± 0.002