Water and Salt - Odyssey Charter School
advertisement

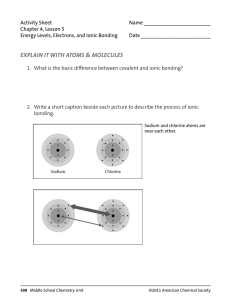
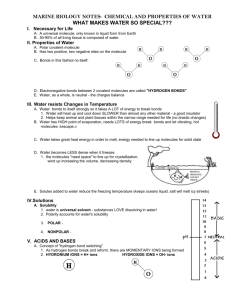
Name___________________ Class_______________ Date__________ Why Does Water Dissolve Salt? Recall our lab on ionic bonding when we looked at salt crystals under a microscope. Salt consists of sodium ions (Na+) and chorine ions (Cl-) joined together through ionic bonding. The following image shows a salt crystal as a result of ionic bonding. Remember, each ion remains charged (positive or negative) even after it’s bonded. Predict: How do you think the polar nature of water molecules will interact with the sodium and chlorine ions? Write a prediction that is at least 2-3 sentences: __________________________________________________________________________________________________________________ __________________________________________________________________________________________________________________ __________________________________________________________________________________________________________________ __________________________________________________________________________________________________________________ Does your prediction match what you know or have observed about salt dissolving in water? Explain: __________________________________________________________________________________________________________________ __________________________________________________________________________________________________________________ __________________________________________________________________________________________________________________ __________________________________________________________________________________________________________________ Work with your partner to complete the following activity: 1. Cut out the ions and water molecules. 2. Arrange the ions on a piece of construction paper to represent a 2-dimensional salt crystal. 3. Arrange the water molecules around the sodium and chloride ions in the correct orientation. The positive part of the water molecules should be near the negative chloride ion. The negative part of the water molecules should be near the positive sodium ion. 4. Move the water molecules and sodium and chloride ions to model how water dissolves salt. Explain what happens when water dissolves salt. Write your explanation below the image. __________________________________________________________________________________________________________________ __________________________________________________________________________________________________________________ __________________________________________________________________________________________________________________ __________________________________________________________________________________________________________________ __________________________________________________________________________________________________________________ __________________________________________________________________________________________________________________ __________________________________________________________________________________________________________________ __________________________________________________________________________________________________________________