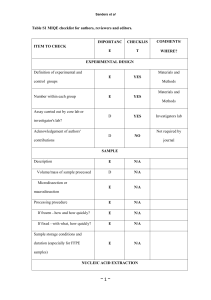
Table S2: MIQE checklist. ITEM TO CHECK IMPORTANCE
advertisement
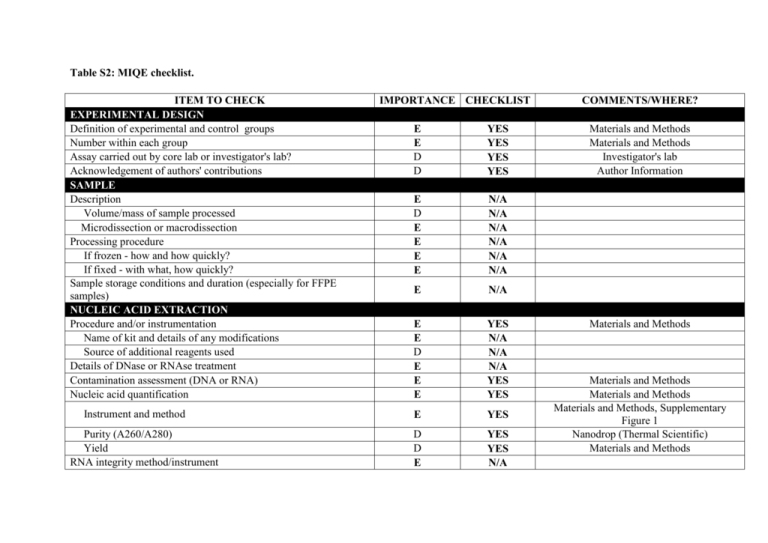
Table S2: MIQE checklist. ITEM TO CHECK EXPERIMENTAL DESIGN Definition of experimental and control groups Number within each group Assay carried out by core lab or investigator's lab? Acknowledgement of authors' contributions SAMPLE Description Volume/mass of sample processed Microdissection or macrodissection Processing procedure If frozen - how and how quickly? If fixed - with what, how quickly? Sample storage conditions and duration (especially for FFPE samples) NUCLEIC ACID EXTRACTION Procedure and/or instrumentation Name of kit and details of any modifications Source of additional reagents used Details of DNase or RNAse treatment Contamination assessment (DNA or RNA) Nucleic acid quantification Instrument and method Purity (A260/A280) Yield RNA integrity method/instrument IMPORTANCE CHECKLIST E E D D YES YES YES YES E D E E E E N/A N/A N/A N/A N/A N/A E N/A E E D E E E YES N/A N/A N/A YES YES E YES D D E YES YES N/A COMMENTS/WHERE? Materials and Methods Materials and Methods Investigator's lab Author Information Materials and Methods Materials and Methods Materials and Methods Materials and Methods, Supplementary Figure 1 Nanodrop (Thermal Scientific) Materials and Methods RIN/RQI or Cq of 3' and 5' transcripts Electrophoresis traces Inhibition testing (Cq dilutions, spike or other) REVERSE TRANSCRIPTION Complete reaction conditions Amount of RNA and reaction volume Priming oligonucleotide (if using GSP) and concentration Reverse transcriptase and concentration Temperature and time Manufacturer of reagents and catalogue numbers Cqs with and without RT Storage conditions of cDNA qPCR TARGET INFORMATION If multiplex, efficiency and LOD of each assay. E D E N/A N/A N/A E E E E E D D* D N/A N/A N/A N/A N/A N/A N/A N/A E YES Sequence accession number E YES Location of amplicon Amplicon length In silico specificity screen (BLAST, etc) Pseudogenes, retropseudogenes or other homologs? Sequence alignment Secondary structure analysis of amplicon Location of each primer by exon or intron (if applicable) What splice variants are targeted? qPCR OLIGONUCLEOTIDES Primer sequences RTPrimerDB Identification Number Probe sequences Location and identity of any modifications D E E D D D E E YES YES YES YES YES YES YES YES E D D** E YES N/A YES YES Supplementary Figure 3 Materials and Methods and Supplementary Table 1 Supplementary Table 1 Supplementary Table 1 Performed previously in Sanders et al., 2011 Performed previously in Sanders et al., 2011 Performed previously in Sanders et al., 2011 Performed previously in Sanders et al., 2011 Supplementary Table 1 Arabidopsis thaliana landsberg variant Supplementary Table 1 Supplementary Table 1 Supplementary Table 1 Manufacturer of oligonucleotides Purification method qPCR PROTOCOL Complete reaction conditions Reaction volume and amount of cDNA/DNA D D YES YES Primers (SIGMA) and probes (ABI) HPLC E E YES YES Primer, (probe), Mg++ and dNTP concentrations E YES Polymerase identity and concentration E YES Buffer/kit identity and manufacturer E YES D E D E D E NO N/A YES YES YES YES Materials and Methods Materials and Methods Materials and Methods, Manufactures proprietory AmpliTaq Gold® DNA Polymerase, UP (Ultra Pure) TaqMan® Gene Expression Mastermix (PN 4369016) Manufactures proprietory Hydrolysis probe quantification only ABI 96-well plates (PN 4306737) Materials and Methods Manual setup Materials and Methods (ABI and Fluidigm) D E E E E D E E E D E YES NO N/A YES YES N/A YES YES YES YES YES Exact chemical constitution of the buffer Additives (SYBR Green I, DMSO, etc.) Manufacturer of plates/tubes and catalog number Complete thermocycling parameters Reaction setup (manual/robotic) Manufacturer of qPCR instrument qPCR VALIDATION Evidence of optimisation (from gradients) Specificity (gel, sequence, melt, or digest) For SYBR Green I, Cq of the NTC Standard curves with slope and y-intercept PCR efficiency calculated from slope Confidence interval for PCR efficiency or standard error r2 of standard curve Linear dynamic range Cq variation at lower limit Confidence intervals throughout range Evidence for limit of detection Supplementary Figure 3 Supplementary Figure 2 No SYBR Green assays used Supplementary Figures 2 & 3 Supplementary Figure 3 Supplementary Figure 3 Supplementary Figure 3 Supplementary Figure 3 Supplementary Figure 3 Supplementary Figure 3 If multiplex, efficiency and LOD of each assay. DATA ANALYSIS qPCR analysis program (source, version) Cq method determination Outlier identification and disposition E YES Supplementary Figure 3 E E E YES YES YES Results of NTCs E YES Justification of number and choice of reference genes Description of normalisation method Number and concordance of biological replicates Number and stage (RT or qPCR) of technical replicates Repeatability (intra-assay variation) Reproducibility (inter-assay variation, %CV) Power analysis Statistical methods for result significance Software (source, version) Cq or raw data submission using RDML E E D E E D D E E D N/A YES N/A YES YES YES No YES YES N/A Materials and Methods Materials and Methods No outliers identified Materials and Methods, Supplementary Figure 2 Standard curve quantification Standard curve quantification Materials and Methods Results Results Not applicable Materials and Methods/Supplemental Materials and Methods/ Supplemental All essential information (E) must be submitted with the manuscript. Desirable information (D) should be submitted if available. If using primers obtained from RTPrimerDB, information on qPCR target, oligonucleotides, protocols and validation is available from that source. *: Assessing the absence of DNA using a no RT assay is essential when first extracting RNA. Once the sample has been validated as DNA-free, inclusion of a no-RT control is desirable, but no longer essential. **: Disclosure of the probe sequence is highly desirable and strongly encouraged. However, since not all commercial pre-designed assay vendors provide this information, it cannot be an essential requirement. Use of such assays is advised against.