Online Supplementary file Mutations in CYP2U1, DDHD2 and GBA2
advertisement

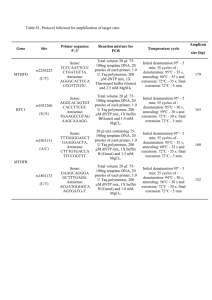
Online Supplementary file Mutations in CYP2U1, DDHD2 and GBA2 genes are rare causes of complicated spastic paraparesis with thin corpus callosum and intellectual disability *1 Andrea Citterio, *1Alessia Arnoldi, 1 Elena Panzeri, 2 Maria Grazia D’Angelo, 3Massimiliano Filosto, Robertino Dilena, 5Filippo Arrigoni, 1Marianna Castelli, 6Cristina Maghini, 6Chiara Germiniasi, 7 Francesca Menni, 8Andrea Martinuzzi, 1,9 Nereo Bresolin,1Maria Teresa Bassi. 4 Scientific Institute IRCCS E. Medea, 1Laboratory of Molecular Biology, 2 Neuromuscular Disorders Unit, 23842 Bosisio Parini, Lecco, Italy; 3Clinical Neurology, Section for Neuromuscular Diseases and Neuropathies, University Hospital “Spedali Civili”, Brescia, Italy ; 4Unit of Clinical Neurophysiology, Fondazione IRCCS Ca' Granda Ospedale Maggiore Policlinico, Milan, Italy; Scientific Institute IRCCS E.Medea, 5 Neuroimaging Unit, 6 Neurorehabilitation Unit, 23842 Bosisio Parini, Lecco, Italy; 7 Pediatric Clinic 1, Department of Physiopathology and Transplantation, Università degli Studi di Milano, Fondazione IRCCS Ca' Granda Ospedale Maggiore Policlinico, Milan, Italy; 8Scientific Institute IRCCS E. Medea, Conegliano Research Center, Conegliano, Italy; 9Dino Ferrari Centre, Neurology Unit, IRCCS Ca’ Granda, Ospedale Maggiore Policlinico Foundation, Dept. of Physiopathology and Transplantation, University of Milan, Italy. Key words: amplicon–based targeted resequencing, spastic paraparesis, CYP2U1, DDHD2, GBA2. Corresponding author: Maria Teresa Bassi PhD, E. Medea Scientific Institute, Laboratory of Molecular Biology, Via D. L. Monza 20, 23842 Bosisio Parini, Lecco, Italy; phone 0039031877111, fax 0039031877499; email mariateresa.bassi@bp.lnf.it, mariateresa.bassi@lanostrafamiglia.it. Online resource Patient and Methods Clinical report of patient P574 with CYP2U1 mutation The proband is the second-born child (aged 4 years and 8 months) of an Egyptian consanguineous family. His elder sister is 6 year-old and is healthy. The mother had previously two miscarriages in the first 3 months of pregnancy. The proband was born at term by caesarean section for fetal distress, but after birth he did not present any sign of asphyxia. His weight at birth was 3540 g. At age 8 months he was admitted to the hospital because of a urinary tract infection: a vesico-ureteral reflux and a right kidney hypoplasia were diagnosed. However after this infection, he did not suffer of any other urinary problems. His developmental milestones were delayed; social smiling appeared at age 3 months while he was able to sit kneeling on his legs at age 1 year. He started cruising although with clumsiness and frequent falls at age 18 months. Babbling started at the age of 8 months. At the first neurological examination (at our clinic) at age 22 months, he showed severe spasticity at the lower limbs, polikinetic deep tendon reflexes, ankle clonus and Babinski sign. At the upper limbs there was only a slight clumsiness of the distal fine movements with slightly brisk deep tendon reflexes. He showed mild drooling, without dysphagia, with normal feeding. Expressive language was limited to two words, although comprehension of simple commands was good. Sitting normally, crawling or walking alone was not possible, although he was able to sit keeping a crouching position and to cruise for few steps with spastic gait using his hands for support. Brain and spinal cord MRI were normal. Brainstem evoked potentials with auditory threshold, visual evoked potentials, somatosensory evoked potentials and electroencephagram were normal. Nerve conduction studies were normal, while needle electromyography showed only slight chronic neurogenic signs. Routine blood test and metabolic screening was normal. At the last neurological examination, the patient (aged 4 years and 8 months) walked only with aid (bilateral support), showing a pareto-spastic gait and a severe spasticity at the lower limb. He had an impaired expressive language development (phonological, morphological and syntactic disorder), with good language comprehension and good global psychological development. The neurophysiology tests were unchanged. Clinical report of affected sibs of the P1242 family with DDHD2 mutation Both sibs of family P1242 (born to not consanguineous parents) showed psychomotor delay that developed into intellectual disability and very early-onset progressive spasticity. In both sibs, onset of the first symptoms of spasticity was before age 1 year. At the time of the first examination in our clinic, the older brother, aged 16 years, was presenting a severe spasticity of the lower and upper limbs with brisk reflexes and moderate weakness associated with mild amyotrophy of the distal portion of the limbs, mild dysarthria, mild drooling, mental retardation and mild cerebellar ataxia. He never acquired ambulation and sphinteric control. At the last neurological examination the patient (age 23) showed a worsening of the clinical condition with severe spastic tetraplegia, trunk ataxia with severe dysarthria and drooling; cognitive decline was evident but he was still able to answer to simple questions. An isolated episode of epileptic seizure had occurred few months before this evaluation. Brain MRI images show severe thinning of the corpus callosum, diffuse reduction of white matter volume and an abnormal hyper-intense signal on T2-weighted and FLAIR images of the residual white matter in fronto-parietal regions. MR spectroscopy was not performed. His younger sister underwent to the first neurological evaluation in our clinic at age 10 years. She presented with a spastic paraparesis with brisk reflexes at both upper and lower limbs, mild distal amyotrophy of distal portion of the lower limbs with mild ataxia and dysarthria. Her walking showed severe unsteadiness and inconstant need of support. Mental retardation was documented through a Wechsler scale (WISC-R) with a verbal intelligent Quotient of 61. Mild behavioral disturbances were reported by the family and were also consistently observed by the neurologist. At the last neurological examination, at age 16 years, the patient showed a more severe spastic paraplegia (able to walk for short routes only with aid), moderate-severe cerebellar signs and more evident behavioral and attention disturbances. Sphinteric control, acquired at the proper time, was maintained. Brain MRI overlaps the picture displayed by the older brother. Strabismus, mild hypomimia, pes cavus and mild scoliosis were observed in both patients. In the two siblings electroneuromyography was normal. Only a mild reduction of motor nerve velocity was observed in the older brother. Motor and sensory evoked potential were presenting increased latency in both sibs. In both sibs metabolic (organic acid and amminoacid, very long chain fatty acid, lysosomial enzymes) and sialotrasnferrin dosage were within the normal range; mutations in mitochondrial DNA were excluded. Clinical report of affected sibs of the P922 family with GBA2 mutation The proband (II-1) is a 24-year-old man who started to complain of difficulty in walking at the age of 10. The disorder has gradually worsened over time and the patient presented progressive stiffness of the lower limbs and frequent falls. The parents are consanguineous and healthy. At the neurological examination lower limb marked spasticity, mild weakness and wasting, brisk deep tendon reflexes, ankle clonus and right Babinski sign were evident. At the upper limbs there were moderate spasticity and brisk deep tendon reflexes with normal strength and no muscle atrophy. He also presented with bilateral eyelid partial ptosis, mild dysmetria, mildly reduced visual acuity and bilateral flatfoot. Although a defined cognitive impairment was not demonstrated, he was a slow learner in school. He also complained of increasing urinary incontinence. Brain and spine MRI was normal as well as electromyography and nerve conduction studies. Motor evoked potentials showed increased central conduction time in all the four limbs. His 24-year-old twin sister (II-2) did not complain of any symptom. She delivered a normal pregnancy at age 19. Clinical examination showed lower limb moderate spasticity, brisk deep tendon reflexes and ankle clonus. At the upper limbs brisk tendon reflexes with positive Hoffman sign were present. Bilateral eyelid ptosis and mild dysmetria were noted. The 22-year-old brother (II-3) also was asymptomatic but neurological examination showed lower limb brisk deep tendon reflexes and ankle clonus. At the upper limbs brisk tendon reflexes and a positive Hoffman sign were present. No significant spasticity was noted. Bilateral eyelid ptosis, mild dysmetria, more evident on the right arm, and bilateral flatfoot were also reported. Amplicon-based high-throughput pooled-sequencing methods Target specific amplicons for the three genes CYP2U1, DDHD2 and GBA2, were prepared by using the sets of primers indicated in supplementary table 1. The primers were designed to amplify by long-range PCR all coding exons and large part of the flanking intronic sequences; the amplicon size ranges between 803 and 3770 bp. Target specific amplicons were processed using a microfluidic platform, the Fluidigm 48,48 Access Array System (Fluidigm Corporation, San Francisco, CA, USA). It allows amplifying 48 specific amplicons from 48 samples in 2304 reaction chambers simultaneously in a reaction volume of 35 nl each. In this pilot experiment we used 48 patients, 46 new and 2 patients mutated one in CYP2U1 (P574) and one in DDHD2 (P1242) as positive controls. Amplification reactions were performed according to the Long Range PCR on the 48.48 Access Array IFC-Advanced development protocol (Fluidigm). Each amplicon library was treated with ExoSAP, while the correct size of the amplicons was checked by using the Agilent DNA 12000 kit (Agilent Technology, Boblingen, Germany). The total DNA obtained for each amplicon library was quantified with Qubit dsDNA BR Assay system (Life Technology Corp., Carlsbad, CA, USA) and processed according to Nextera XT DNA sample Preparation protocol (Illumina, San Diego, CA, USA). Briefly, each sample was tagmented (tagged and fragmented) by the Nextera XT transposome and then PCR amplified by adding index primers required for clusters formation. PCR products were then purified by using the AMPure XP beads (Beckman Coulter, Milano Italy); this provides a size selection step that removes very short library fragments from the population. Each library was normalized and pooled for MiSeq (Illumina) loading. Data analysis was performed by using MiSeq Reporter and Integrative Genomics Viewer (IGV) v. 2.3.20. At least 93% of the NGS fragments had an average coverage of 399 reads and passed quality control. The remaining 7% that did not pass the quality control were re-sequenced by traditional Sanger sequencing. Overall, all amplicons from the three genes were produced with the exception of the amplicons 1 of CYP2U1 and GBA2 genes (corresponding to exon 1 for both genes); due to the lack of PCR product these amplicons did not produce any cluster. Exon 1 for both genes was sequenced by Sanger method. Only DDHD2 mutation of patient P1242 (previously identified by Sanger sequencing) was correctly identified also by targeted resequencing (Supplementary Figure 1). The CYP2U1 mutation located in exon 1 was not detected due to the failure of long range amplification of exon 1 (amplicon 1) of the gene. A mutation in exon1 3 of GBA2 was identified (Supplementary Figure 1) and confirmed by Sanger sequencing as described in the result section. Online resource Figure 1 Legend Partial schematic view (by IGV software) of the reads alignment encompassing the mutations identified by targeted resequencing in exon 13 of GBA2 (P922) and in exon 16 of DDHD2 (P1242) genes. Online resource Figure 1 Online resource Table 1 Primer sets used for long range amplification Primer Name Sequence CYP2U1NGS-amp1F AAG GGA AAC CAA CAG CCA ATC AG CYP2U1NGS-amp1R CTT CTT CTG TGT TTT CTG GAC TT CYP2U1NGS-amp2F AGG GCT GCT GCC GAC AAC AG CYP2U1NGS-amp2R CAT CTT ACC AAC TCC CTA TGA GTT CYP2U1NGS-amp3F GAA GAA TAC AGG GAG GAT GGT G CYP2U1NGS-amp3R CAT TTA GAT GAG CTC TTC TGA CCT CYP2U1NGS-amp4F GTC AGC TGG TAA GAG CCA GG CYP2U1NGS-amp4R CAC GAC AGA GTC CAC TTC ATC DDHD2NGS-amp1F GTT CTC AGC ACC CAG AAG CAG DDHD2NGS-amp1R GGC TTT CGG CTC CAT TGT CC DDHD2NGS-amp2F TGT TGC AAT CTA GTT TCG AGC ATG DDHD2NGS-amp2R DDHD2NGS-amp4F CTT TAC AAA CCG TCA AAC CAT TCC GAA TAT AAC TCA GAA TTG TAT GTT GGG CAA GGA GTT ATT ACC AGG TAT AAA TTT CTA CTT GAC TAG AAA TTT ATA CCT GG DDHD2NGS-amp4R TTA GCC AGT TAG AAT AAG ACA CCC DDHD2NGS-amp5F GGG TGT CTT ATT CTA ACT GGC TAA DDHD2NGS-amp5R DDHD2NGS-amp7R CAT CTG AAG ATG GAA ATG GTT ATA CA TTA AGC TTG AAG GCT TAA AAT TGG AAG CCA AGA ATG ACA GAG ATA ATT AAA AGT C GAC TTT TAA TTA TCT CTG TCA TTC TTG G CTG GTT AGT CTG CTC CAT TGC T DDHD2NGS-amp8F AGC AAT GGA GCA GAC TAA CCA G DDHD2NGS-amp8R ATC CCA CAA AGC ACA AGG ACA C DDHD2NGS-amp9F AGC ATT ATC TTC CCA CAG TGC C DDHD2NGS-amp3F DDHD2NGS-amp3R DDHD2NGS-amp6F DDHD2NGS-amp6R DDHD2NGS-amp7F Amplicon Size (bp) Exon Amplicon Number 1333 EX1 Amplicon 1 1741 EX2 Amplicon 2 1860 EX3 Amplicon 3 2198 EX4-EX5 Amplicon 4 EX1 Amplicon 1 2371 EX2-3 Amplicon 2 3401 EX4-5 Amplicon 3 2658 EX6 Amplicon 4 1554 EX7 Amplicon 5 EX8-EX9 Amplicon 6 1621 EX10 Amplicon 7 1834 EX11 Amplicon 8 998 803 DDHD2NGS-amp9R GGT TGT TGG CAT CAT TAG CCT CA DDHD2NGS-amp10F GGA GGG AGA CTT CGT TAC TTT CT DDHD2NGS-amp10R TTG AGA CGG GTG AGT CCA GG GBA2NGS-amp1F CAA CCT GAG CTC TGC CTC AG GBA2NGS-amp1R CTT ACT CCC TTA ATA TGG TAG CTG GBA2NGS-amp2F GGA AAC CAT AAA TGG TCC TTT CAG GBA2NGS-amp2R CTG AAC TTT GGT GAA GGT TCC TC GBA2NGS-amp3F CTC TGA AAG GGG AGG AGC TG GBA2NGS-amp3R CAT GGA AAC AAG GCC TAC TTA TTA C GBA2NGS-amp4F GTA ATA AGT AGG CCT TGT TTC CAT G GBA2NGS-amp4R CTA GGC TCT TCC CAG ACA CC GBA2NGS-amp5F AAC TTC TGT GGT CCC TCA GAT TG GBA2NGS-amp5R CAC TTA ATG GCT TTC TGG GTC TTT 2763 EX12-EX13-EX14-EX15-EX16 Amplicon 9 3770 EX17 Amplicon 10 2964 EX1 Amplicon 1 1187 EX2-EX3 Amplicon 2 1556 EX4-EX5-EX6 Amplicon 3 1764 EX7-EX8-EX9-EX10-EX11EX12 Amplicon 4 2405 EX13-EX14-EX15-EX16-EX17 Amplicon 5