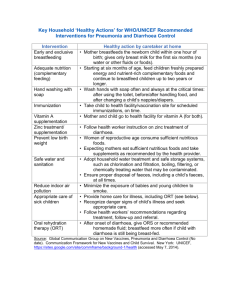
New diagnostics, drugs and vaccines for diarrhoeal disease and
advertisement

New diagnostics, drugs and vaccines for diarrhoeal disease and malaria - PATH 2013-18 Business Case Research and Evidence Division Human Development Team May 2013 PATH Product Development Business Case Intervention summary What support will the UK provide? DFID will provide £16,050,000 over the period 2013-14 to 2017-18 to PATH, for Product Development research to develop new diagnostics, drugs and vaccines for diarrhoea, and a new G6PD test for vivax malaria treatment to prevent relapse. DFID’s funding will support the development of the most promising candidates to contribute to the improvement of health in developing countries. Why is UK support required? Diarrhoea and malaria are two of the leading causes of severe and life-threatening illness in the poor, especially in children. Prevention and treatment of diarrhoea have historically received significantly less attention than in other diseases. Vivax malaria, the predominant form in South Asia, has received much less attention than falciparum malaria. There are no theoretical reasons vaccines to prevent several of the major causes of diarrhoea cannot be developed and deployed, reducing incidence of disease and transmission. Better treatment for diarrhoea would help reduce mortality; most children who die or are permanently harmed by diarrhoea do so from dehydration. With better treatment that is available in the periphery patient outcomes should be significantly improved. The majority of vivax malaria is recurrent malaria (relapse). There are drugs which can prevent these relapses by killing the dormant forms in the liver, but both the current drug (primaquine) and its successor (tafenoquine) cause potentially lifethreatening haemolysis and anaemia in people who have the genetic enzyme deficiency G6PD deficiency. There are tests available for this in high-resource settings, but they require laboratories not available in low-resource settings. Since between 5% and 15% of people in malaria areas have G6PD deficiency this means the drugs to prevent relapse cannot be used. Getting a cheap near-patient test would be a massive step towards controlling vivax malaria. There are market failures around the development of new technologies for diseases of poverty. In the years between 1975 and 2000, only 13 new drugs were registered for use for neglected diseases (approx. 1% of the total new drugs). With increased investment by Governments, Donors, Philanthropic Foundations and others, there have been 43 new products registered in the last 10 years with a further 350 in development. These products, including drugs, vaccines and diagnostics for a wide range of diseases (e.g. malaria, sleeping sickness, visceral leismaniasis, pneumonia, diarrhoea, meningitis, encephalitis, TB), have the potential to save many hundreds of thousands of lives What are the expected results? Support to PATH will, in the long term, contribute to the impact goal of reduced poverty through the improved prevention, diagnosis and treatment of two leading causes of death and illness among the poor – diarrhoea and malaria. The shorter term, specific outcome will be to take innovative and lifesaving technologies to scale in low-resource settings. 2 PATH Product Development Business Case Expected outputs include: WHO prequalification of at least 2 rotavirus vaccines Advancement of bacterial diarrhoea vaccines to Phase 3 trials Industrial partnerships for diarrhoea vaccine manufacturing in emerging economies New oral rehydration therapies for the management of diarrhoea approved and launched in 3+ countries New drugs for treatment of 3 leading causes of childhood diarrhoea (Shigella, Cryptosporidium, and soil-transmitted helminths) ready for clinical trials Field evaluation of late-stage prototype diagnostics for determining diarrhoeacausative agents (and enabling correct treatment) completed Performance and appropriateness of all currently available G6PD tests evaluated in order to support correct vivax malaria treatment in populations where G6PD deficiency is common 3 PATH Product Development Business Case 1 Strategic Case A. Context and need for a DFID intervention Significant progress has been made in the last two decades in improving the health and preventing deaths of people living in poverty. For instance, between 1990 and 2011, the mortality rate in children under 5 years fell from 84.1 to 52.8 deaths per 1,000 live births and amongst women in the same period, maternal deaths have fallen from over 400,000 per year to under 275,000.1 However, this progress has not been evenly spread either geographically or between the rich and the poor, and over 7 million women and children still die every year, many during pregnancy and birth: the great majority from easily treatable or preventable conditions such as diarrhoea and malaria. Inequalities persist within and between countries and both health and life expectancy among those in the poorest quintiles are worse almost everywhere.2 Few low income countries are expected to achieve MDGs 4 and 5 at current rates of progress.3 In some cases we urgently need new tools, technologies and knowledge to prevent deaths and reduce mortality and in other cases the knowledge and the tools exist to prevent many of these deaths. New technologies and innovations are making faster progress possible (for example, more effective vaccines, longer lasting bed-nets and more stable and cost-effective medicines). A strong evidence base is essential for the provision of more effective development and humanitarian assistance to the poorest. We need new knowledge to improve the ways in which we currently work, cease doing things that are ineffective, and make sound decisions between competing priorities. This not only involves identifying new drugs, devices and interventions but also new and better ways of putting them into practice. The Secretary of State reaffirmed DFID’s commitment to strengthening the evidence base in her first major speech on development in 2012, stating: I want to make sure that we invest in what works. Where we don’t know, I want to find out… [I] will make sure we are clearer about where we should focus our resources so we know what …. will actually work as we intend” 4 This programme will focus on developing and using new technologies for diarrhoeal disease and malaria. Diarrhoeal diseases More than 800,000 children less than five years old die each year from severe, dehydrating diarrhoea or dysentery (approximately 11% of total childhood deaths), 1 Lozano, R. et al, Progress towards Millennium Development Goals 4 and 5 on maternal and child mortality: an updated systematic analysis, The Lancet, Volume 378, Issue 9797, Pages 1139 - 1165, 24 September 2012. 2 Gwatkin, D., Wagstaff A., &Yazbeck, D., Reaching the Poor with Health, Nutrition and Population Services: What works, what doesn’t and Why, The World Bank, WashingtonDC, 2005 3 Just 9 out of 137 developing countries worldwide are set to achieve both Millennium Development Goals (MDGs) 4 and 5 to improve the health of women and children. The remaining 128 developing nations will fall short. Based on current trends, 31 developing countries worldwide will achieve MDG 4 (to reduce the under-5 mortality rate by two-thirds between 1990 and 2015) and 13 countries will reach MDG 5 (to reduce the maternal mortality ratio by three-quarters during the same period). – cite – Lancet September 20, 2011. 4 Rt Hon Justine Greening, Speech to the Conservative Party conference, Tuesday, 9 October 2012 4 PATH Product Development Business Case and millions more are hospitalised5,6. Many more suffer from malnutrition associated with diarrheal disease and its long-term adverse consequences on physical and cognitive development. The leading causes of diarrhoea in infants and young children are Cryptosporidium, cholera, rotavirus, and the bacterial infections of Shigella and enterotoxigenic Escherichia coli (ETEC). Combined, these pathogens are responsible for a significant portion of childhood diarrheal disease and deaths, which mainly occur in the developing world. In resource-poor areas many children lack access to appropriate medical care for severe diarrhoea, sanitation and safe water sources are inadequate or non-existent, and malnutrition increases the risk of disease. While a long-term focus on all interventions is critical, vaccines offer the best near-term and most equitable approach to drastically reducing the incidence and severity of diarrhoea. Two commercial rotavirus vaccines exist, and their effectiveness and impact on reducing deaths and hospitalisations has been demonstrated in countries where they have been introduced, including low-resource settings like Mexico. However, these vaccines remain expensive and the production capacity of their manufacturers cannot meet global demand. As a result, they are not yet widely available or affordable for low-resource populations without support from donor agencies. In order to create and maintain a financially sustainable and healthy market, additional manufacturers, especially those in emerging countries, must enter the marketplace. Despite Shigella and ETEC being long-term World Health Organisation (WHO) vaccine targets, there are no currently licensed vaccines against these pathogens. This is due to inadequate investment and a lack of a significant commercial market in the developed world. However, given their ongoing contribution to deaths and nutritional deficits among children under the age of five, Shigella’s increasing resistance to antibiotics, and the challenges to provide universal improved sanitation and access to clean water, the need for vaccines against Shigella and ETEC is critical. Development of these new vaccines will lead to fewer deaths and have a broad impact on children and families. Vaccines to prevent diarrheal diseases may reduce malnutrition associated with severe diarrhoea in children. They can also help break the cycle of poverty in poor communities, because, even with treatment, diarrhoea causes a significant morbidity burden. The most direct effects are seen on physical and cognitive development and perhaps other long-term problems such as irritable bowel disease and autoimmune disorders. Lost time from work or school due to frequent diarrheal illnesses has an impact on education and income, and the prevention of disease can positively impact educational rates and local economies. Regardless of cause, the common symptom of fluid loss in paediatric diarrhoea means that the replenishment of lost fluids and electrolytes via Oral Rehydration Salts (ORS) is the cornerstone of treatment. In illness due to cholera infection, the onset of diarrhoea is rapid, and if left untreated, the mortality rate is 50% to 60%, with death occurring within hours to days. However, with immediate appropriate 5 Fischer Walker CL, Perin J, Aryee MJ, Boschi-Pinto C, Black RE. Diarrhea incidence in low- and middle-income countries in 1990 and 2010. BMC Public Health. 2012;12(220). 6 Liu L, Johnson HL, Cousens S, et al. Global, regional, and national causes of child mortality: an updated systematic analysis for 2010 with time trends since 2000. The Lancet. 2012;379(9832):2151–2161. 5 PATH Product Development Business Case treatment, the mortality rate can be reduced to less than 1%7. ORS consists of electrolytes (sodium and potassium chloride) and glucose, which promote water absorption, and costs only a few cents per treatment. ORS is actively promoted by the World Health Organisation (WHO) and the United Nations Children’s Fund (UNICEF) and is credited with saving millions of lives worldwide since its introduction in the 1970s8. A combined prevention and treatment strategy that includes vaccines, oral rehydration solution, zinc treatment, exclusive breastfeeding, and improved hygiene, sanitation, and nutrition, has a significant impact on diarrhoea morbidity and mortality. However, efforts to increase their use have faced many challenges, including limited access to and awareness of the interventions, weak country-level systems to implement them, and complacency about diarrhoea. ORS has been the subject of countless public health campaigns, demographic and health surveys suggest that nearly 90% of mothers in Africa and Asia know about ORS, but less than 30% use it9. Greater uptake and coverage of ORS can be achieved by collaborating with private-sector partners to develop new formulations and presentations of these relatively simple products that meet the expressed needs of the market. Malaria diagnostics In many low-resource settings, access to appropriate diagnostic technologies is severely lacking, and health care providers often resort to presumptive treatment of many illnesses, with potentially serious consequences. Misdiagnosed patients are exposed to unnecessary medication and associated side effects, additional financial costs, or—even worse—higher rates of mortality. For malaria, diagnostics are an important part of realising the full potential of current and emerging antimalarial drugs to reduce the global burden of malaria and bring the disease closer to elimination. Plasmodium falciparum and P. vivax are the most common species of malaria parasites infecting humans. P. vivax is the most widely distributed and there are 2.5 billion people at risk of infection with P. vivax and an estimated 80 to 300 million clinical cases every year10. Only one currently available drug, primaquine, effectively clears parasites from the human body, thus avoiding relapse. Primaquine also kills the form of the malaria parasite that the mosquito needs to transmit infection to other people. There are some safety issues with the use of primaquine (and next-generation drugs like tafenoquine), which belong to the 8-aminoquinoline class of drugs. Patients who have a mutation in the gene for an enzyme (G6PD) can suffer from a break down of their red blood cells (the severity depends on the degree of enzyme deficiency) if they take medication from this class of drugs. 7 Sack DA, Sack RB, Nair GB, Siddique AK. Cholera. The Lancet. 2004;363(9404):223–233 t Sack DA, Sack RB, Nair GB, Siddique AK. Cholera. The Lancet. 2004;363(9404):223–233 9 Forsberg BC, Petzold MG, Tomson G, Allebeck P. Diarrhoea case management in low- and middle-income countries: an unfinished agenda. Bulletin of the World Health Organization. 8 10 Malaria: burden and interventions. DFID Evidence Paper 2010 6 PATH Product Development Business Case The G6PD deficiency affects more than 400 million people worldwide and an appropriate diagnostic test is required to enable the safe, widespread use of primaquine and similar drugs. Although there is clear recognition of the need for more appropriate diagnostic tests to determine a patient’s G6PD status, there is very little information available to understand the market for malaria-related use of G6PD testing. There is also a lack of operational data on how these tests can be implemented to ensure safe administration of primaquine and related antimalarials. There is no clear guidance about regulatory and quality standards that tests can be evaluated against to mitigate the risk of adoption of poor-quality tests. What will the DFID Funding support? PATH is an international non-profit organisation with a mission to improve the health of people around the world by advancing technologies, strengthening systems, and encouraging healthy behaviors. PATH programmes work in partnership with local communities and a range of partners to take an entrepreneurial approach to developing and delivering highimpact, low-cost solutions, from lifesaving vaccines and devices to collaborative programmes with communities. PATH works in more than 70 countries and together with partners with the aim of empowering people to achieve their full potential. PATH programmes take a number of approaches to move solutions from innovation to impact: supporting new ideas through inception, development, and testing; paving the way for introduction in low-resource countries; and working with governments and communities to integrate and expand the most successful ideas. Solutions are developed in collaboration with the communities who will use them, together with a range of other partners, including community groups, non-governmental organisations, governments, companies, and United Nations agencies. PATH has a number of different areas of work: emerging and epidemic diseases, new technologies, maternal and child health, reproductive health and vaccines and immunisation. Twenty technologies worked on by PATH (for example affordable meningitis vaccine, better systems for malaria bednet distribution, integrated strategies for using new vaccines, uniject – technology for safe injections) are currently sold and in distribution globally or regionally. Ten are in global use and have had a significant impact on policy, practice, or local capacity for innovation and production. Senior managers within PATH oversee all programmes, to ensure that work is coordinated across the organisation. PATH has a set of impact initiatives, including malaria, maternal and neonatal health technologies, and diarrheal disease/pneumonia. These impact initiatives have set clear, aligned, and aspirational goals, with accompanying objectives and indicators to guide a significant proportion of PATH’s work in priority health areas. The DFID funding will cover three different PATH teams who together work across vaccine development (diarrhoea), drug discovery (diarrhoea) and diagnostics (malaria and diarrhoea): 7 PATH Product Development Business Case Enteric Diarrhoeal Disease group (EDD) OneWorld Health (OWH) Diagnostics Group (DxG) Enteric Diarrhoeal Disease Group (EDD) – Diarrhoea Vaccines The work of this group includes the development of low cost, field-adapted, vaccines for two leading causes of diarrhoea – rotavirus (RV) and bacterial infections (Shigella and E.coli) – in resource-poor settings. Working with industrial partners in both China and India, over the next 5 years they intend to 1) Submit 2-4 RV vaccines for WHO pre-qualification (numbers dependent on phase 3 trial results), 2) work with emerging country manufactures to ensure low-cost vaccine production, 3) advance their pipeline of vaccines against Shigella and E.coli through early stage development and 4) test new ways to administer vaccines (e.g oral or intradermal). One World Health (OWH) – Diarrhoea treatments and diagnostics OWH are the only PDP developing new treatments for paediatric diarrhoea and work across three areas – oral rehydration, drugs and diagnostics. Over the next 5years they hope to 1) develop new oral rehydration salt (ORS) formulations and presentations and ensure their approval and launch in at least three countries, 2) advance a pipeline of new drugs for 3 major causes of diarrhoea through pre-clinical and early clinical development, and 3) complete field evaluations of late-stage development diagnostic prototypes to enable identification of the diarrhoea-causative agent and thus correct treatment. They will do this through three main mechanisms : - Drug discovery partnerships and collaborations - Efforts to strengthen local capacity to conduct high-quality, cost-effective clinical trials in low-income countries Engaging in strategic communications to raise awareness and adoption of diarrhoea diagnostics and treatments in underserved populations Diagnostics Group (DxG) – Malaria Diagnostics The diagnostics group has a range of projects, focused on accelerating the development of affordable and appropriate diagnostic technologies specific to a number of disease areas, facilitating their introduction into low-resource settings and supporting their appropriate and correct use in the field. The funding from this programme will be used to support work to evaluate the currently available G6PD diagnostic tests for their performance and appropriateness to support malaria treatment and elimination. Work will include: 1. Developing demand-side readiness to evaluate and adopt G6PD tests 2. Strengthening the market environment for the supply of G6PD tests 3. Developing G6PD test products that best align with target product profiles 4. Building the evidence base for G6PD testing through demonstration studies Alignment with wider DFID priorities The Coalition Government has taken a global leadership role, with major pledges of support for the treatment and control of malaria and neglected tropical diseases. For these disease control programmes to be effective there is an urgent need for new diagnostics, drugs, vaccines and other technologies. DFID Ministers have also 8 PATH Product Development Business Case reaffirmed their commitment to the use of evidence and new technologies in DFID’s work in a number of speeches11. The research work of the PATH teams is strongly aligned with DFID’s wider strategic priorities, contributing to the pillars for action set out in the UK’s Framework for Results for malaria12 and reproductive, maternal and newborn health13. Supporting the rapid development of health technology innovation is a key strand of DFID’s Structural Reform Plan (SRP) Pillar 1 – International commitments to support actions to achieve the MDGs relevant to health and education. The technological innovations also support SRP Pillar 5 – The role of women, in particular to improve maternal health and access to family planning. The development of new technologies is one of the three pillars of DFID’s research strategy, across all areas of work, not just health. There is high level international political support of for the development of new health technologies, and this is one of the UK commitments through the G814. Fit with Research and Evidence Priorities DFID research has three main aims: 1) Developing new technologies and innovations that have impact on poverty or the effects of poverty; 2) Finding better and more cost-effective ways of delivering development to those who need it, including stopping things that do not work; and 3) Better understanding of key development questions and background to support best policy choices. This programme fits well with DFID’s research and evidence priorities and is about 1) developing new technologies and 2) finding better and more cost-effective ways to deliver development interventions. B. Impact and Outcome that we expect to achieve Support to PATH will, in the long term, contribute to the impact goal of reduced poverty through the improved prevention, diagnosis and treatment of two leading causes of death and illness among the poor – diarrhoea and malaria. The shorter term, specific outcome will be to take innovative and lifesaving technologies to scale in low-resource settings. Investments in PATH will deliver high quality and cutting edge research that will improve our understanding of the development of new technologies for prevention, diagnosis and treatment of diarrhoeal disease and malaria diagnostics. Specific outputs include: WHO prequalification of at least 2 rotavirus vaccines : Advancement of bacterial diarrhoea vaccines to Phase 3 trials 11 Rt Hon Justine Greening Speeches 1) to Conservative Party conference, Tuesday, 9 October 2012; 2) Open Up! Using technology to build open societies and make aid smarter Speech 13th November 2012 and 3) Development in transition Speech on 7 Feb 2013 12 13 http://www.dfid.gov.uk/malaria http://www.dfid.gov.uk/What-we-do/Key-Issues/Health/Reproductive-maternal-and-newborn-health/ 14 July 2005 the Gleneagles G8 communiqué on Africa committed to increase direct investment and taking forward work on market incentives through public private partnerships and AMCs. This commitment has been retained as the G8 pledges have been updated. 9 PATH Product Development Business Case Industrial partnerships for diarrhoea vaccine manufacturing in emerging economies New oral rehydration therapies for the management of diarrhoea approved and launched in 3+ countries New drugs for treatment of 3 leading causes of childhood diarrhoea (Shigella, Cryptosporidium, and soil-transmitted helminths) ready for clinical trials Field evaluation of late-stage prototype diagnostics for determining diarrhoeacausative agents (and enabling correct treatment) completed Performance and appropriateness of all currently available G6PD tests evaluated in order to support correct malaria treatment in populations where G6PD deficiency is common 2 Appraisal Case A. What are the feasible options that address the need set out in the Strategic case? A recent RED review of research models15 identified six basic models in common use across DFID that aim to deliver a complementary mix of high quality relevant research, facilitate research-into-use and strengthen capacity to undertake and use research: 1. Provision of core funds to international research organisations 2. Product Development Partnerships (PDPs) awarded following open competition 3. Research Programme Consortia (RPCs)16 awarded following open competition 4. Direct funding for defined areas of research (other than to RPCs), awarded following open competition 5. Funding relationships with UK Research Councils (UKRCs) 6. Collaboration with other bilateral donors, for programmes with shared objectives The work described in this business case falls under the second mode, Product Development Partnerships (PDPs). A number of options were explored to secure the development of new products for neglected diseases of poverty. Four funding options, including the do-nothing counterfactual, are considered below. Within the wider health portfolio other work is supported using some of the other models of research funding listed above. Option 1: The “do nothing” counterfactual Under this option DFID would not invest in product development research leaving the development of new health technologies, for diseases that affect the poorest, to the private sector and/or other research funding bodies. 15 J. Piper, Working Paper, Research and Evidence Division, March 2010 RPCs are centres of specialisation with partners from high and low income countries working together to explore a set of research questions around a common theme e.g nutrition, health systems, reproductive health 16 10 PATH Product Development Business Case Option 2: Direct funding to stimulate the pharmaceutical industry Under this option DFID would seek to provide direct support to industry to attempt to stimulate them to develop and ensure access to health products for diseases of poverty. Option 3: Direct funding to academic R&D institutions Under this option DFID would seek to provide direct support to academic R&D labs to develop and ensure access to health products for diseases of poverty. Option 4: Invest in a portfolio of health product candidates through the PDPs housed at PATH (the preferred option) Under this option DFID would invest in the three PDPs housed in PATH to develop new diagnostics, drugs and vaccines for malaria and diarrhoeal disease, which, through open competition, have already been identified as a market leader in this field. Product Development Partnerships (PDPs) are non-profit entities that act as virtual pharmaceutical companies, coordinating the development of new health technologies and drawing upon the respective strengths of academia, large and small industry at different stages of the R&D and commercialisation process Options appraisal Option 1: the do-nothing counterfactual This was the default position until the inception of Product Development Partnerships (PDPs) in the late 1990s and early 2000s. As indicated in the strategic case, prior to the formation of PDPs and significant public investments in global health product R&D, few new treatments for neglected diseases of poverty were coming on stream. There was a lack of incentives for the private pharmaceutical industry, due to the high costs of new drug development and the extremely limited profit margins associated with making them available to patients in need. In the years between 1975 and 2000, only 13 new drugs were registered for use for diseases of poverty (~1% of the total new drugs)17. The argument for investing in drug development is based on the lack of viable markets for the product of that research (i.e. new medicines, diagnostics and technologies) as well as acknowledged market failures around the commercial investment for R&D related to NTDs. The main factor explaining the lack of supply of research in general is the global public good nature of knowledge and research18. Research and development is a 17 Moran M. et al London School of Economics and Political Science, The New Landscape of Neglected Disease Drug Development Wellcome Trust 2006 18 A public good is a good that is both non excludable and non-rival, in that individuals cannot be effectively excluded from use and where use by one individual does not reduce availability to others. Global public goods are public goods which are non-rival and non-excludable throughout the whole world, as opposed to a public good which exists in just one national area. Knowledge and research is the classic example of a global public good. 11 PATH Product Development Business Case public good and some of the products of that research may also have public good characteristics (e.g. vaccines). Traditionally, Intellectual Property (IP) rights have been used to provide incentives for the private sector to invest in risky and expensive R&D programs by enabling monopoly pricing of pharmaceutical products. The biotechnology and pharmaceutical industries rely heavily on IP portfolios to protect their investments, provide signals to the investment community which encourage future investment, provide incentives to enter a market (rather than wait) and to justify prolonged expenditures in view of regulatory risk and market uncertainty. Research funded by private pharmaceutical companies is done with the principal aim of developing commercial products that can be protected by patents and licensing arrangements and sold at a profit. However, the ability of industry to exploit IP with respect to neglected diseases is severely limited in large part because the effective demand for those products will be limited, without government intervention. This is because those suffering from these diseases are poor meaning they will be prepared to pay only a low price for treatment. When the cost of R&D is high and there is insufficient potential for profit to justify the necessary investment, then there is no incentive for the private sector to invest, which is especially the case for late stage clinical trial research. Moreover, all treatments and other products for diseases – regardless of whether the sufferer is poor or not - display the classic attributes of a ‘merit good’19 meaning that individuals often underestimate their benefits and don’t consume enough. Theory predicts that although markets may emerge to supply some merit goods, it will be insufficient to achieve a socially efficient level of consumption. While a number of factors explain the lack of demand for NTD products in general (i.e. presence of externalities, information inequities and the divergence between private and social benefits), the most relevant for diseases of the poor is the fact that those suffering from these diseases are not able to pay the full market price and will under consume. This lack of commercial return is a critical stumbling block that stops industry from undertaking research for new products for neglected disease singlehandedly. The challenge can only be overcome by sharing costs and risks with research partners. With increased investment by Governments, Donors, Philanthropic Foundations and others, there have been 43 new products registered in the last 10 years with a further 350 in development. These products have the potential to save many hundreds of thousands of lives20. In 2000, a report21 identified that only 10% of global funding for health research was devoted to the conditions that represent 90% of the global disease burden (the so called “10-90 gap”). The PDPs housed in PATH are an example of a global public good. They are developing new health technologies, for those that are too poor to be able to pay enough for them to make it worthwhile for pharmaceutical companies to invest the 19 A merit good is is a commodity which is judged that an individual or society should have on the basis of some concept of need, rather than ability and willingness to pay. 20 Saving lives and creating impact: EU investment in poverty related neglected diseases. Policy Cures/DSW 2012 21 Global Forum for Health Research Report 2000. The 10/90 report on health research. Geneva. 12 PATH Product Development Business Case huge amounts required in the R&D process to develop and produce them. This is particularly the case given the steep rises in costs and complexity of developing pharmaceutical products seen over the years. Estimated out-of-pocket expenditure now ranges between US$403 – US873 million for drug development22 and as costs have increased the number of products in development has fallen. While R&D spending by large pharmaceutical companies doubled between 2000 and 2010 approvals of new drugs (mainly for high income country application) fell significantly23. There is a clear market failure and it is readily accepted that public investment is required to incentivise activity in neglected disease drug research and development. In 2007 it was estimated that R&D on new drugs for neglected diseases of the poorest countries required an additional US$6-10 billion over the next ten years24. Data from the 5th annual G-FINDER Report (for product development research spending in 2011) showed that while the public sector currently provides nearly $2billion (two thirds of all funding), for neglected diseases R&D, at the global level government funding has continued to move away from product development and towards more traditional, earlier stage, basic research, while industry or philanthropic investments in this area have become either smaller and/or more focussed on only a narrow range of diseases and products. If this trend continues it may mean that the public sector funds only basic research, leaving product development to industry or philanthropy and resulting in a subsequent lack of new medicines, vaccines or diagnostics for neglected diseases reaching the market25. This analysis suggests that the do-nothing counterfactual is not a viable option. There are a number of International goals, to deliver new technologies for diseases of poverty by 2018 and the associated higher level impact goal of ensuring the achievement of global eradication and elimination targets. The ‘do-nothing’ counterfactual has therefore been rejected and is not considered further. The three remaining options all seek, in different ways, to stimulate R&D for new products for neglected diseases of the poor. Option 2: Direct funding to stimulate the pharmaceutical industry One way to drive product development for neglected diseases R&D is to try to address the market failure by trying to stimulate the private sector to invest in this area. In recent years an increasing number of push and pull mechanisms have been proposed in order to incentivise the pharmaceutical industry to develop more health technologies (e.g. drugs, vaccines and diagnostics). Push mechanisms such as subsidies for research, tax credits for R&D, expedited regulatory review are outside the scope of DFID’s work. Subsidies for research and tax credits have been implemented in some countries but have not made an appreciable difference to stimulating private sector activity in this area specifically. Funding through PDPs, Paul SM et al (2011) How to improve R&D productivity: the pharmaceutical industry’s grand challenge. In Nature Reviews: Drug Discovery iv10:328-329 23 David E et al (2010) New frontiers in pharma R&D investment. In McKinsey Quarterly. 24 Dalberg Global Development Advisors (2007) Initiative for Sustainable Financing for R&D for Neglected Diseases. Available at: www.ifpma.org 25 Policy Cures (2012) Neglected Disease R&D: A Five-Year Review: The Fifth Annual G-Finder Report. 22 13 PATH Product Development Business Case another push mechanism, is discussed under Option 4. Pull mechanisms include Advanced Market Commitments and Prize Funds. These ‘pull’ mechanisms can be used to bring-in private sector investment and risk bearing, at different points in the development, manufacturing and delivery value chain. Advanced Market Commitments (AMCs) AMCs are designed to use existing incentive structures within the pharmaceutical R&D model. An AMC works by providing industry with financial incentives to develop new technologies for neglected diseases, as would be the case for high prevalence conditions in high income countries, reproducing the expected economic returns for a new product as viewed at the start of the R&D process (the time when the investment decision is made) as opposed to at the time of sale. The financial incentives are market guarantees, where the investors (likely donors, philanthropists or governments) legally agree to buy a minimum quantity of the product to be developed at a set price, thus assuring a minimum market size and profit margin to industry. The first AMC, developed for pneumococcal vaccines (worth US$1.7billion), was launched in February 2009, with investors (including DFID and a number of other donors) promising to buy (and supply through GAVI) 2 billion doses of vaccine in low- and middle-income countries by 203026. When the AMC was introduced there were already two pneumococcal vaccines in late stages of development, and a recent report by GAVI27 highlights that the AMC acts primarily as an incentive to scaling up production capacity and as a procurement mechanism for nearly developed products rather than a R&D incentive. There have been no AMCs for early stage product development and their application is limited. They are unlikely to be applicable in the case of diseases such as sleeping sickness where the remaining global disease burden is relatively small (limiting the potential market) and often associated with fragile states (potentially limiting market reach) but where the need for new drugs and diagnostics is pronounced. Prize Funds An alternative model to stimulate private sector R&D for health technologies for neglected diseases is prize funds. Examples include the Health Impact Fund and the Medical Innovation Prize Fund, where cash rewards are offered either for the development of new health technologies or for achieving measurable health impacts occurring as a result of the availability of new technologies. This approach changes the existing incentive structure for R&D, shifting the R&D driver towards patient impact (and thus the health products that will have the largest health impact per pound invested) rather than economic return on investment, and may result in increased efficiencies in the R&D process, competitors seeking to lower their operating costs in order to maximise potential profit. Unlike AMCs, prize funds could 26 Lahte J (2012) Is the pneumococacal vaccine advance market commitment motivating innovation and increasing manufacturing capacity? Some preliminary answers. In Vaccine v30(14): 2462-6 27 Cernuschi, T et. al. “Pneumococcal Advanced Market Commitment: Lessons Learnt on Disease and Design Choices.” GAVI White Paper. Sep, 2011. www.gavialliance.org/library/gavidocuments/amc/monitoring-and-evaluation/pneumococcal-advance-market-commitment--lessonslearnt-on-disease-and-design-choices-and-processes 14 PATH Product Development Business Case theoretically target the development of multiple drugs for multiple diseases through a single channel. While theoretically promising (and supported by a number of prominent economists) prize funds are currently untested and there are a number of practical reasons why they are unlikely to be an effective investment mechanism for developing drugs for neglected diseases at this point in time: a) The proposals suggest that prize fund payments would be tied to measures based upon available data relating to reported use of medicines, outcomes and approval processes while currently little such data exists creating real challenges to determining payment models; b) To be effective the prize funds need to be large (suggestions range from a minimum of US$6 billion per year to a high of US$ 240 per year) and currently there appears to be little political will amongst potential donors to invest in an internationally managed funder over which they would have little control. This is evidenced by the widespread lack of support (from either donors or industry) for either the Health Impact Fund or the Medical Innovation Prize Fund in their current form28; c) In common with AMCs, while not yet proven, it is likely that prize funds would disproportionately skew R&D towards later stage development. Whichever pull mechanism is used to attempt to stimulate industry to work on technologies for neglected diseases, one of their key advantages is that they shift the risk from the funder to the R&D partner who only receives payment once the technology has been successfully developed. However, as indicated by the pneumococcal vaccine AMC investment pot and the proposed necessary budgets for effective prize funds, the financial ‘pull’ incentive may need to be very large, particularly for those technologies that are earlier in the R&D cycle, scientifically more complex to develop or associated with other substantial hurdles to industry engagement (e.g. lack of expertise in conducting trials in developing countries). It is difficult to judge, in advance, the optimal size of pull incentive required – creating a real danger of either overpaying, in which case there is an element of unnecessary subsidy to industry, or underpaying in which case the incentive will be ineffective. Where a major pharmaceutical company is involved in developing products for diseases of poverty, it is typically driven by corporate social responsibility (CSR) objectives and, in some cases, because developing countries may be major markets and important sources of revenue in the future. The CSR programmes of major pharmaceutical companies usually revolve around their expertise and existing products, currently aimed at the developing world. Often these projects rely on single champions, with no corporate urgency to accelerate the project through the development process, and focused on developing single candidates29. This focus, instead of a building a portfolio of compounds or searching for new compounds, increases the overall risk of failure and also has the impact of decreasing efficiency and increasing costs. Gold RG & Marin J-F (2012) Ibid http://www.forbes.com/sites/sarikabansal/2011/11/22/top-five-ways-big-pharma-neglecteddiseases/ 28 29 15 PATH Product Development Business Case Option 3: Direct funding to academic R&D institutions While direct funding to industry might be one way to stimulate pharmaceutical activity in later stage R&D for neglected diseases much of the more basic research required takes place in academic laboratories where more of the global scientific expertise in tropical medicine is situated. One of the key strengths of academics working in global health drug development is their early innovation and demonstrated capability and productivity in early stage discovery (new disease targets and chemical compounds), a strength coupled with a strong scientific reputation (gained largely through publication in peer-reviewed journals) which allows them to effectively engage with the regulatory authorities and influence later-stage development by other stakeholders. While often highly successful at leveraging both public and private sector funding and managing R&D costs within their grant parameters, academic institutions tend to take a very project-based approach, working on extremely narrow product development activities rather than taking a larger pipeline or portfolio approach. This can both increase risks of failure (the costs of failure being borne by the funder) and limit their ability to utilise promising early-stage chemical entities for other potential uses. They can also lack the ‘translational’ capabilities to ensure that promising new chemical entities and/or drug (or other technology) candidates can be turned into appropriate formats to address patient need and/or manufacture and distribute these. R&D that takes place in academic institutions is often slower and more fragmented (with activities being conducted in an interrupted fashion alongside other priority activities) than in either the pharmaceutical industry or efforts led by Product Development Partnerships (PDPs)30 To enable combinations of drugs to be developed (to avoid resistance to new drugs by the pathogens) there would still need to be direct oversight by some organisation over the disease portfolio as a whole. This could be problematic, where academic research groups typically oversee work on one or a small number of potential candidates and could be met with reluctance by investigators who value their autonomy. Opportunities to integrate the clinical and basic programs could be lost and there may be duplication of investment to develop facilities that a more coordinated approach could share. Option 4: Invest in a portfolio of product development R&D through support to PDPs (preferred option) Jointly created and funded by governments, private foundations and industry, PDPs work like “virtual” pharmaceutical companies with a small, central group of core staff coordinating the development of a portfolio of new health products (e.g. vaccines, drugs, diagnostics) by drawing on the respective strengths of academia, large pharma and smaller biotech companies at various stages of the product development cycle. While academic R&D is driven by the potential for high profile, peer reviewed publication, and industrial R&D by profit, as non-for-profit entities, PDPs are primarily driven by patient need. 30 Moran M. et al London School of Economics and Political Science, The New Landscape of Neglected Disease Drug Development Wellcome Trust 2006 16 PATH Product Development Business Case The portfolio approach diversifies risk, increases the likelihood of developing combination therapies, decreases overall risk of failure and can reduce costs through such factors as platform approaches, the screening of compound libraries for applicability against multiple diseases, selection of only the most promising candidates for later stage development, and the leveraging of not only finance but technical expertise and infrastructure from a range of different partners. Careful financial oversight within PDPs allows funding to be redistributed, when individual projects are stopped, if they do not meet their milestones. They work on all stages of the product development cycle from basic research through to registration, faster and at lower cost than either the academic or private sectors31. Over the past 10 years PDPs have been successful in the development of a wide range of new health products for use in developing countries. During this time there have been 43 new products registered with another 350 in development, PDPs being responsible for approximately half of this 32. The pharmaceutical industry has been a particularly active and willing partner and in recent years (2007-11) the proportion of funding for neglected disease R&D from the pharmaceutical industry has been rising. The private sector currently contributes 13% of the total spending33. Representatives from the pharmaceutical industry have highlighted that PDPs play an instrumental role in breaking down the institutional barriers between partners who otherwise would not work together and allowing for R&D developments that would not previously have been possible. In addition in areas where there is little or no commercial return, the private sector view the role of the PDP as critical to avoid duplication and to focus on the most promising candidates, wherever they originated from. Acting as neutral, coordinating bodies, in many ways the funding of a PDP to deliver new drugs for neglected disease represents a combination of options 2 (funding to industry) and 3 (funding to academia), allowing DFID to capitalise on the strengths of each partner. Funding PDPs enables DFID to support a wide portfolio of R&D activities via a single organisation (rather than via a number of smaller contracts for individual activities with different partners). The neutrality of PDPs (and lack of profit drive) enables them to develop strong relationships with developing country governments and the appropriate (global and national) regulatory agencies which increases their ability to conduct clinical trials in disease endemic environments and to ensure the timely certification, registration and use of new drugs. Product Development Partnerships have a range of different IP agreements with their pharmaceutical, and other, partners, using the guiding principle of developing global public goods34, i.e. that access to treatments should be equitable and 31 Moran M (2005) A Breakthrough in R&D for Neglected Diseases: New Ways to Get the Drugs We Need. In PLoS Medicine v6 32 Policy Cures (2012) Saving Lives and Creating Impact: EU Investment in Poverty-related Neglected Diseases. 33 Policy Cures (2012) Neglected Disease R&D: A Five-Year Review: The Fifth Annual G-Finder Report. 34 Changing with the times – doing the right things in the right places in the right way – presentation to DFID EMC May 2013 17 PATH Product Development Business Case treatments should be affordable to those who need them. Where the PDPs do not control the IP related to each of their products they negotiate appropriate licensing rights (e.g. non-exclusive and sub-licensing rights) to enable them to deliver products to patients at cost in low income countries. In some cases the private sector partner may be able to commercialise in high income countries, if a market exists. The private sector has a history of sharing IP with PDPs, and acknowledges that they will not commercial returns in this area of their work. For some products (e.g. vaccines for TB and HIV) modelling studies have identified a potential commercial market, once products are available. These studies highlight the need for push funding in the near future, with the potential for differential pricing at later stages, and the funds realised can then be used to support future developments35. PDPs collaborate with the public and private sectors in developing countries to improve capacity building for research into product discovery, development and manufacture. The technology transfer can lead to sustainable production of new technologies and result in new jobs being created in developing countries. The main identified risk associated with funding PDPs relates to the issue of funding sustainability. Drug, vaccine and diagnostic development is lengthy and uncertain leading to a need for stable long-term financing. However, PDPs are largely funded by the public and philanthropic sectors whose funding tends to be short-term (five years and under) and unpredictable. In addition some funding can be earmarked, that is provided for specific projects This lack of flexibility can prevent PDPs from being able to use and move funds strategically across their portfolios to the most promising projects36. However, the PDPs do have safeguards in place to protect against these risks through ensuing they have diversified funding sources and maintain a balance of core versus earmarked funding. Concluding rationale for preferred option: funding the three PDPs based at PATH The below table presents a quantitative comparison of the three options discussed against a series of 5 critical success criteria. Building on the options appraisal above, each option is scored between 1 (low/poor) and 5 (high/good) for each of these criteria and then summed to give an overall score. Beneath the table we present a succinct narrative to explain why, on the basis of these criteria, funding to the PDPs at PATH is the preferred option Compared to either industry or academia alone, PDPs have very strong recent history of delivering effective, safe, affordable and, critically, appropriate technologies (including vaccines and diagnostics) for diseases of the poor. They have a strong pipeline of new potential products, pointing to the likelihood of continued high performance through to 2018. 35 Aeras and TBVI: TB Vaccine Research and Development: A Business Case for Investment: 2013 Hecht R, Wilson P & Palriwala A (2009) Improving Health R&D Financing for Developing Countries: A Menu of Innovative Policy Options. In Health Affairs v28(4): 974-985 36 18 PATH Product Development Business Case Critical Success Criteria Able to deliver effective, safe, affordable and appropriate neglected diseases to those populations that need them most Ability to bring together the right partners Costs are proportionate and manageable Ability to manage risk of failure Leveraging of wider resources Total Score Option 2 (fund pharma) score 4 Option 3 (fund academia) 3 Option 4 (fund PATH PDPs) 5 3 3 5 2 3 4 3 2 4 1 2 4 13 13 22 PATH's PDPs have the ability to broker partnerships across the full range of players necessary to ensure successful product development (from basic science through to delivery and post implementation surveillance), which is demonstrated through their strategically chosen partners (including industry and academia as well as other PDPs). The partnership model allows them to limit core costs and approach product development in a flexible and cost-effective manner which results in significantly lower costs for development than seen in the private sector, and faster lead times than seen in either industry or academia. By adopting a portfolio of activities across a range of diseases and ensuring a balance between core and earmarked funding, PATH PDPs are also well-placed to minimise the risk of failure. In order to support this strategic approach to portfolio management, DFID proposes to continue to core fund the PATH PDPs. The strong decision-gates mean that only the most promising candidates move from earlier to later stage development, both reducing risks of failure and increasing operating efficiencies and value for money. B. Assessing the strength of the evidence base for each feasible option In contrast to many development interventions, there is limited published evidence comparing the effectiveness of different funding models for delivering new products. Therefore it is not possible to present formal evidence ratings for each option using the DFID standard criteria. Instead, the decision on which funding model to use is based largely on qualitative assessments and critiques of different models for stimulating global health product development37, external evaluations of the various PDPs operating in the global health space, the personal viewpoints of other stakeholders (including other R&D funders and pharmaceutical partners), and DFID’s own judgement and expertise in managing research. This is in line with recent guidance from the Quality Assurance Unit38. 37 38 Gold RE & Morin J-F (2012) Promising Trends in Access to Medicines. In Global Policy v3(2): 231-237 QAU Report Violence against Women and Girls’ Research and Innovation Fund. See p.28 19 PATH Product Development Business Case Previous research39 suggests four reasons why PDPs are particularly effective in securing increased industry engagement to accelerate product development: PDPs have skills and resources, such as infectious disease expertise and access to developing country clinical trial sites, that companies may lack PDP funding is crucial to the development of a ‘no profit no loss’ model for big pharma, particularly at the stage of large scale clinical trials, making ‘public good’ activities more acceptable in board rooms and to shareholders. PDPs act as a ‘push incentive’ as they significantly reduce R&D costs for industry because of sharing costs with the PDP; PDPs can provide an important source of revenue for smaller industry partners, either as a commercial contractor of services or as a grant funder for technology research (often without diluting equity as venture capitalists would). PDPs can also help broker partnerships with larger companies that may be needed to undertake large clinical trials or scale up manufacturing processes. This may include technology transfer to and partnerships with developing country pharmaceutical companies; and PDPs have been successful in raising the issue of future access to new products by developing countries with donors and potential recipient countries. Addressing access issues may be difficult and politically sensitive for companies to take on by themselves (as they are seen as having vested commercial interests). However, it is essential for industry engagement that mechanisms are in place for any successful new product to be used. Evidence ratings for the options discussed in the business case Option Evidence rating 1 2 3 4 Do nothing Weak Weak Medium C. For Each Feasible Option, What is the Assessment of Local Capacity? Is the Intervention Likely to Strengthen Capacity in a Durable Manner? The development of products for neglected diseases necessitates the conduct of clinical trials in disease-endemic countries where research capacity is often weak. The pharmaceutical industry can find it particularly challenging to enter and work in these contexts, where governments can be wary of large pharmaceutical companies and where poor in-country infrastructure can make accessing research sites and conducting trials particularly difficult. While academics may find it easier to enter these countries to conduct research they often lack access to pre-established clinical trial sites and associated laboratory facilities. PATH programmes have a presence in a number of developing countries, and have, over the years, invested heavily in the development of local R&D capacity (both in terms of infrastructure and staffing). 39 Moran M. et al London School of Economics and Political Science, The New Landscape of Neglected Disease Drug Development Wellcome Trust 2006 20 PATH Product Development Business Case Where possible they leverage previous investments in capacity-building through the conduct of multiple trials at single sites. D. What is the likely impact (positive and negative) on climate change and environment for each feasible option? Categorise as A, high potential risk/opportunity; B, medium/manageable potential risk/opportunity; C, low/no risk/opportunity; or D, core contribution to a multilateral organisation The climate change and environmental impacts are likely to be similar for all three feasible options (though it is possible that negative impacts will be slightly reduced via the PDP option due to enhanced operating efficiencies associated with the collaborative way of working with partners). A single climate and environment assessment (CEA) is presented here. Option Climate change and environment risks and impacts, Category (A, B, C, D) Climate change and environment opportunities, Category (A, B, C, D) 1 Do nothing 2 Support commercial research group 3 Develop new partnerships 4 Support PDPs C B C B B B B B The activities that will be implemented/funded as part of this intervention are unlikely to have any major environmental impact or involve any serious/substantial risks to environmental and human health. However there are still a number of issues associated with all kinds of medical research that need some level of consideration and adequate management. The disposal of medical and/or bio-hazardous waste is one of them as improper disposal can have very serious impacts on the environment, ecosystems and human health40,41,42. Responsible environmental management is included in various international and regional standards and regulatory requirements such as those set by the International Conference on Harmonisation of Technical Requirements for the Regulation of Pharmaceuticals for Human Use43 and in the WHO Good Laboratory and Good Manufacturing guidelines44. Such guidelines further lay strict standards for the use of animal and human subjects in research as initially laid out in the World Hossain MS et al (2011) Clinical solid waste management practices and its impact on human health and environment--A review. In Waste Management v.31(4):754-66. 41 Johannessen LM et al (2000) Health Care Waste Management Guidance Note. World Bank. Washington. 42 Prüss A et al (2000) Safe management of wastes from health-care activities. WHO. Geneva. 43 See www.ich.org for further information 44 See http://www.who.int/medicines/areas/quality_safety/quality_assurance/production/en/ for documentation 40 21 PATH Product Development Business Case Medical Association’s 1964 Helsinki Declaration. PDPs and their partners reflect these strict international safety standards, as well as national environmental standards and regulations, in their own Standard Operating Procedures (SOPs) which include environmental impact reduction (in particularly associated with waste disposal). Where disease endemic country partners do not have existing SOPs, DNDi and other PDPs provide technical support to develop these. New drugs and associated medical products will not pass through the WHO or national regulatory process without demonstrating strong adherence to international, regional and national standards, and the European Medicines Evaluation Agency (EMEA) in particular now requires a full environmental risk assessment for all new medical devises. Additionally, all vaccine, drug and diagnostics trials must obtain ethical approval both from their host institutions and within the countries within which they are conducted; such approval will include adherence to national environmental and safety legislation. To this end all PDPs have or are developing environmental policies and safety guidelines in place. Over the funding period covered by this business case DNDi will be collating some of these in the form of an Environmental Charter which will apply to themselves and all their partners. From an operational perspective, PDP operations are likely to have significant carbon footprints, particularly associated with international travel for meetings, workshops, conferences and field activities. As business class flights are about three times more carbon intensive per flight than for economy seats45, DFID will also recommend that as much travel (associated with this funding) as possible is economy, irrespective of staff seniority, unless there are specific reasons to the contrary. Of the work supported by this business case, the diarrhoea portfolio needs the most extensive considerations: Most diarrhoeal pathogens, including rotavirus, Shigella, E.coli, and ETEC are transmitted via the faecal-oral route and can be spread through the environment and drinking water supplies. In many developing country contexts rates of access to improved sanitation and safe drinking water are low, increasing the risk of environmental faecal contamination (through open defaecation) and exposure. The development of diarrhoeal vaccines will act to reduce the risk of infection, while new diagnostics and drugs will allow for timely and effective treatment; together advances across these three areas will contribute to reduced risk of environmental contamination through the prevention and effective management of infection. This will become increasingly important in areas likely to experience increased temperatures and rainfall (including more intensive rainfall events), as a result of climate change, these two climatic factors (especially when coupled with increasing housing density in the urban sector) resulting in conditions more favourable for the spread of diarrhoeal pathogens46. 45 46 UK Department for Forestry and Rural Affairs (2009) WHO (2009) Protecting health for climate change: connecting science, policy and people. 22 PATH Product Development Business Case The main potential environmental risks associated with this portfolio relate to the use of attenuated live vaccines and associated pathogen shedding in the environment. However, there are already two live rotavirus vaccines licensed and in use in many countries, with no demonstrated environmental risks detected47. In the case of the live Shigella and ETEC vaccines under development, PATH are ensuring that they are developed in such a manner that they do not compete well in the environment (soil, sewage or surface waters) and will not pose an environmental risk in terms of pathogen shedding. PATH will continue to monitor potential environmental risks associated with the development and use of live diarrhoeal disease vaccines but the current evidence suggests minimal threat. While this funding envelope is restricted to supporting vaccine, diagnostics and drugs, PATH also houses a ‘Technology Solutions Global Programme’ which is exploring innovative ways to improve access to safe water through water storage and treatment technologies. All teams working on diarrhoeal diseases across the organisation are represented on and work together via a cross-team working group to ensure a fully comprehensive approach to diarrhoea prevention, management and control, including environmental interventions (water and sanitation). PATH have established the www.defeatdd.org website to provide a full range of diarrhoeal disease control tools for advocates, documents and guides for programme officers and links to further information on interventions to control diarrhoea, and they continue to work with governments across the globe to develop integrated diarrhoeal disease control programmes that include both medical and environmental interventions. E. If Any, What are the Likely Major Impacts on Social Development? Neglected infectious diseases (NIDs), including diarrhoea and malaria, affect the poorest, hardest to reach people, who often live in remote or conflict-affect areas with minimal access to health services. Part of the rationale for eliminating these disease is to help break the poverty cycle and improve economic prospects for the poor. Preventing morbidity from NIDs will reduce out-of-pocket expenditures on treatment, reduce time caring for sick children and other family members and contribute to increased school attendance and productivity. The development of new products to tackle NIDs will have a disproportionate impact on the health and economic productivity of the poorest, most marginalised peoples across the globe. F. For Fragile and Conflict Affected Countries, What are the Likely Major Impacts on Conflict and Fragility, if Any? N/A G. What are the Likely Costs and Benefits of Each Feasible Option? Identify the preferred option. DFID Business Cases require a cost-benefit analysis to be conducted for each feasible delivery option. This requires the identification and quantification of 47 Anderson EJ (2008) Rotavirus vaccines: viral shedding and risk of transmission. In The Lancet v8(10): 642-649 23 PATH Product Development Business Case monetary value to costs and benefits. Applying a formal cost-benefit analysis to research and development investments is difficult. Whilst a description of the required characteristics (target product profiles) are developed for each desired new product a) it is difficult to predict early in the product development what the precise final drug efficacy and/or costs will be and b) it is beyond the scope of the product developer to ensure that drugs produced reach all patients in need of treatment. There is limited publically-available evidence on the relative cost-effectiveness of PDPs versus industry models. This is driven in part by the commercially-sensitive nature of industry data, but even more so by the reality that most of the commercial pharmaceutical industry now conducts the majority of their neglected disease R&D activity through partnerships with PDPs, rather than alone in-house. Given the data challenges is not possible to perform a full cost-benefit analysis of the options discussed in this business case. Such analysis would be based on many assumptions and lack credibility. There is some evidence that suggests the PDP model is a cost-effective mechanism for neglected diseases product R&D, especially when compared with standard commercial product development48. The study reviewed the work of drug development PDPs, which together had a combined portfolio of 40+ projects, representing 75% of all identified on-going product development (at the time of the study). The researchers identified that R&D was more fragmented and slower in the academic sector than the private sector and through PDPs and also estimated that the cost of bringing a new drug to market via a PDP at that time was US$150 million compared to US$800 million via an industrial pharmaceutical company working alone. This discrepancy in costs reflects a combination of factors including the ability of PDPs to leverage substantial in-kind inputs from a large range of partners (an unpublished analysis by one of the PDPs suggests that for each $1 of public investment they are able to leverage $1.5-$2.5 from the private sector). The same analysis suggests that R&D is slower and more fragmented in the academic sector than in the private sector or when coordinated by PDPs49. Given the analysis and the associated risks outlined above, the judgement is that Option 4 DFID provides funding for the PDPs housed at PATH is the preferred option. Comparing core cost components across different delivery mechanisms Commissioning and managing a programme of product development entails four broad cost categories: Core Costs - including central management, monitoring and research and dissemination of information and overhead costs. Implementation Costs - including resource materials, equipment costs, experimental research and development activity, travel expenses and consultants fees. DFID staff time – the costs of DFID oversight and on-going management 48 Moran M. et al London School of Economics and Political Science, The New Landscape of Neglected Disease Drug Development Wellcome Trust 2006 49 Moran M et al (2006) The New Landscape of Neglected Diseases Drug Development. Wellcome Trust. 24 PATH Product Development Business Case External evaluation – including costs of assessing the impact of the programme The table below briefly and quantitatively compares these different cost categories across the three options considered in this business case (not including the counter-factual). Core Costs Option 2: Direct support to pharma research High: costs include support to facilities and resources and complex management structures Implementation Costs High: done to international regulatory approval standards, based in developed countries DFID staff time Medium: likely to be high in first years, including commercial expertise from corporate departments and technical expertise from across DFID. Likely to be high level of staff input to ensure coordination of activities with other partners External evaluation High: Could be very sensitive when dealing with Option 3: Direct support academic research Medium: includes basic science lab costs, project based with facility and resource costs included. UK Universities claim full economic costings and others are often >20% Medium: but may not be done to regulatory approval standard High: likely to be high in first years, including commercial expertise from corporate departments and technical expertise from across DFID. In addition further staff time required to set up robust M&E and other systems to capture baselines, outputs and outcomes. Likely to be high level of staff input to ensure coordination of activities with other partners Medium: likely to include a number of different partners if Option 4: Support to PATH PDPs Low: largely virtual organisations leveraging in support using other’s facilities and resources with small staff and simple management structures. Overheads charged at 13% High: done to international regulatory standards, but often in partnership with developing country partners where costs may be slightly lower Low: building on existing structures within PDPs that have been set up explicitly to manage technical and access partnerships and M&E systems. Low: PDPs activities are more transparent although some 25 PATH Product Development Business Case Option 2: Direct support to pharma research commercial information Option 3: Direct support academic research each individual academic group is working on specific projects Option 4: Support to PATH PDPs commercial in confidence sensitivities. Opportunities to do (and demand for) joint evaluations with other donors. Expected benefits The broad benefits of the programme components are set out in the Strategic Case. The benefits in terms of research are new and improved health technologies for diseases of poverty, leading to a range of health, social and economic benefits for women, girls, men and boys. The benefits from investing in the PATH programmes to develop new products for malaria and diarrhoeal diseases are best considered in terms of: 1. Creating and maintaining a long-term, financially sustainable supply of rotavirus vaccine The development and introduction of new, affordable vaccines against the leading causes of severe diarrhoea in low-resource settings could lead to drastic reductions in the rates of disease, death, and long-term negative impacts on children and their families. For rotavirus vaccines, demand in the developing world is outpacing supply. This gap is expected to reach 20 million doses by 2015 and will expand as additional countries seek to introduce the vaccines. By filling the gap between supply and demand, with low cost vaccines, there could be another 60 million vaccine courses added to the global supply by 2020, with an estimated lowering of vaccine prices to less than US$1 per dose. It is estimated that this will avert 700,000 to 1 million deaths among infants and children under five years50. 2. Developing treatments for diarrhoea Developing novel treatments targeted to work against specific pathogens will reduce the duration of infection and limit the spread of diarrhoeal diseases in the population. Affordable and accessible new treatments for diarrheal disease have the potential to directly reduce poverty among the world’s poorest people by increasing economic productivity as well as reducing deaths and illness among children. Improving the formulation, packaging, and presentation of ORS will increase and expand its use. ORS is credited with saving millions of lives since its introduction in the 1970s by reducing mortality associated with acute secretory diarrhoea. Yet research shows that it is only used in an estimated 34% of cases of paediatric diarrhoea in developing countries51. 50 Atherly DE, Lewis KD, Tate J, Parashar UD, Rheingans RD. Projected health and economic impact of rotavirus vaccination in GAVI-eligible countries: 2011-2030. Vaccine 2012; 30(1 Suppl): A7-14. 51 Gupta GR. Tackling pneumonia and diarrhoea: the deadliest diseases for the world's poorest children. The Lancet 2012; 379(9832): 2123-4 26 PATH Product Development Business Case 3. Safe and widespread adoption of treatment options for malaria (in particular P. vivax) Approximately 8% of the population in malaria-endemic countries have G6PD deficiency, (some studies have recorded local prevalence as high as 28%). This corresponds to an estimated 220 million males and 133 million females prone to adverse events resulting from primaquine and tafenoquine treatment. The safe and wide-scale adoption of these antimalarial treatments to accelerate progress toward malaria elimination relies on the availability of low-cost, point-of-care diagnostic tests for G6PD deficiency. Several tests are commercially available, but few have been rigorously evaluated, and most are not practical for use at the point of care for malaria case management. Quantifying the benefits of research Although it is not possible to quantify the full societal and economic benefits arising from funding global health research, there is strong suggestive evidence from the UK that it is likely to be high. In 2008, the Medical Research Council, the Wellcome Trust and the Academy of Medical Sciences commissioned a study to develop methodology and calculate the economic benefits of public and charitably funded medical research in the UK52. The report, Medical Research: what's it worth?, demonstrated that the return on investment for public and charitable funding of medical research was substantial. The estimate for cardiovascular disease research for instance was that for every £1 invested once, a stream of benefits worth 39p every year over the long term was generated. The health gains in the period 1985 - 2005 from clinical interventions relevant to cardiovascular disease were worth £53bn to the economy. The proportion attributable to UK public and charitable cardiovascular disease research was estimated at around 20%, and the time taken for research to be translated into practice averaged 17 years. The internal rate of return from public and charitable investments in cardiovascular disease research was therefore about 9%, a further 30% was contributed from spill-over effects to and from the private sector. In the UK the net value to the UK of health gains and ‘spill over’ benefits arising from medical research has been shown to wield a significant impact on the UK economy53. There are as yet no equivalent estimates for the economic return on investment for global health research. H. Theory of change for the preferred option 52 Medical Research: what is worth? science/WTX052113.htm 53 Impact of MRC research: October 2010 http://www.wellcome.ac.uk/About-us/Publications/Reports/ Biomedical- 27 PATH Product Development Business Case As previously stated, DFID’s investment in the PDPs based at PATH will allow them to continue to contribute to reducing the global burden of neglected diseases and improving the quality of life for patients and populations at risk. PATH’s theory of change for product development partnerships is shown above. Product development partnerships are an effective model that leverage the strengths of partners—commercial, academic, government, research, and/or nonprofit—to develop global public health goods that are appropriate, available, accessible, and affordable. A PATH document, Maximising the benefits of public-private partnerships, has been developed to describe the framework and case studies. The work of the PATH PDPS leads to the following high-level outputs: 1. Improved product development pipeline: through the use of best practices in portfolio/project/quality management, resources are efficiently applied toward development of products with the most promise to make global health impact. This leads to: 2. Increased supply of safe and effective products: the outputs of a robust product development pipeline are proven products that meet the global public health requirements (e.g., appropriate, affordable). Products are manufactured in adequate supply to meet needs. 3. Improved enabling environment at global and national levels: the enabling environment consists of supportive health policy and guidelines; defined regulatory pathways; adequate financing/market incentive for product development, introduction and scale-up; and development of capacity to validate, introduce, and deliver new products. This leads to: 4. Improved support from global and national stakeholders: products pass public health and regulatory review. Sustainable financing and delivery plans are developed. Stakeholders approve and support product introduction and scale-up. 28 PATH Product Development Business Case 5. Improved product adoption environment: the targeted health issue is a high priority among the target beneficiaries. Products are appropriate for target populations and designed for use in low-resource settings. This leads to: 6. Increased consumer and market demand for products: consumers and health workers are aware of and demand new products. Mechanisms and support for procurement and distribution are established. These outputs lead to the outcome of increased uptake of lifesaving products. Effective and safe global health products demanded and implemented at large scale will contribute to a reduction of morbidity and mortality in low-resource settings. I. What measures can be used to assess Value for Money for the intervention? PATH take a patient-needs approach to R&D and work with a diverse range of partners to ensure the most appropriate and cost-effective approach to the development of new health technologies. ICAI (2011)54 suggests that to represent value for money, programmes receiving support from DFID should: Ensure they have realistic and appropriate objectives and a clear plan for achieving their intended impact, Have robust delivery arrangements to support the desired objectives and demonstrate good, transparent governance and management through the delivery chain, Demonstrate a transformational, positive and lasting impact on the lives of the intended beneficiaries, Incorporate learning to improve future aid delivery. PATH’s PDPs have an ambitious set of objectives and their past performance and proven pathways to impact suggest these targets remain realistic. Their patientneeds driven approach to portfolio development ensures they remain focussed on the development of the most appropriate health technologies to have maximal transformational impact on the lives of the poorest. They have strong and transparent governance structures in place, including clear stop/start gates for decision-making about which products to take forward in the product development pipeline, to support the delivery of their objectives. Their role as facilitator, with the objective of empowering local partners with contributions such as needs assessments, seed or core funding, knowledge transfer, administrative and business development support also acts to both keep costs down and maximise transparency and accountability to intended beneficiaries in disease endemic countries. Their partnership model approach allows them to keep down core costs while ensuring robust delivery through a network of appropriate partners along different stages of the R&D process. This same model allows them to operate as neutral brokers, giving them strong legitimacy among all partners including disease-endemic country governments. They continue to explore different partnership and business models to assess their feasibility and impact. In order to ensure investments in PATH’s PDPs continue to represent good value for money for DFID there are a core set of indicators to be reported annually. These are 54 ICAI (2011) ICAI’s Approach to Effectiveness and Value for Money. 29 PATH Product Development Business Case presented in the table below, categorised according to the core VfM indicators of economy, efficiency, effectiveness and cost-effectiveness. Criteria Economy – getting the best value from inputs Assessed Attributes Increased technical capacity among PATH’s partners in low- and middle-income regions Efficiency – maximising the outputs for a given level of inputs Increased capacity and conduct of clinical trials for new products Increased evaluation and registration of new products Effectiveness ‒ ensuring that the outputs deliver the desired outcome PDP-driven outcomes Specific Measures Number of partners in lowand middle-income regions able to manufacture vaccines according to international standards (ARVAC) Number of partners in lowand middle-income regions able to conduct preclinical work to international standards (DxG) Number of new vaccines and mucosal adjuvants, using cGMP manufacturing processes, developed and ready for transfer to partners in low- and middle-income regions (EVI) Number of manufacturers engaged in diagnostic product development (DxG) Number of clinical trial sites ready to conduct international standard trials (DxG) Number of clinical trials implemented, number of patients included in studies, number of patients treated outside of the studies (EVI, ARVAC, DxG) Number of INDs filed with USFDA (DxG) Number of in-country evaluations of diagnostic products (DxG) Number of new treatments registered Number of new chemical entities registered (DxG) Progress towards WHO prequalification of vaccine candidates (ARVAC) Number of vaccines studied in target population in expanded Phase 2 studies 30 PATH Product Development Business Case Criteria Assessed Attributes Costeffectiveness Expansion of funders and leveraging of donated funds by PDPs Specific Measures with final vaccine formulation made to commercial scale (EVI) Number of countries expanding radical cure of P. vivax. (DxG) Progress towards Millennium Development Goal #4 (DxG) Number and diversity of partners and funders (EVI, ARVAC, DxG) Leverage of donated funds against inputs/activities by other organisations toward same outcome.) PATH’s measures to assess value for money are applied at different stages of the PDP development and implementation process. Initially, in the consideration of any new product development, a target product profile (TPP) is developed. All potential candidate technologies are then assessed against this for their scientific and health potential. PATH-led PDPs seek the opinion of independent, international expert reviewers as well as internal scientific opinion, using the following criteria: Scientific potential: What are the prospects for good scientific progress? Is the concept scientifically sound? What is the background to the scientific basis of the work; is there supportive evidence from previous work to indicate that the project is headed in the right direction? What are the experience and qualifications of partners, particularly in developing countries; is the collective expertise adequate to ensure all technical areas are covered? Are there any regulatory obstacles that would likely prevent this product from becoming licensed? Fit with the TPP: Will the candidate be able to fulfil the profile described? Is the technology agile enough to adjust to potential minor changes in the TPP? Is the cost of the product within an acceptable window for end-users? Potential to increase local capacity: Can the PDP be used as an effective and efficient mechanism to increase local capacity to evaluate, manufacture, and/or use the technology in low- and lower-middle-income countries? Justification for resources: Can PATH and the PDP justify the funds requested as essential for the work and it is supported by the scientific potential on the scale requested? Does the PDP-specific product proposal represent good value for money? Once the determination is made to move forward with a technology or product, PATH and its individual PDPs monitor spending and value for money throughout the lifetime of the individual research projects and have a robust process if further 31 PATH Product Development Business Case resources are required. Down-selection criteria are clear and predefined to prevent the use of additional resources on a product that is unlikely to become a licensed product. Subcontractor performance is routinely evaluated and technical assistance provided as needed to ensure regulatory and performance standards are met. PATH PDPs utilise TPPs to guide decision-making at significant points in their programs. Also critical to PATH’s success is the reliance on a lean virtual model to leverage appropriate expertise without onerous overhead. Each of PATH’s PDPs use risk-adjusted development strategies to build and manage their portfolios of early-stage projects (based on new development leads) and late-development-stage projects (e.g., re-purposing candidates within the Drug Development Group). The specific PDP portfolios and TPPs are used to determine project-specific prioritisation and execution. Once project-specific activities reach the phases for clinical evaluation or implementation, PATH executes the programs through their well-established network of clinical trial and program implementation sites. PATH’s network of partners and sites ensures that they conduct high quality research using good clinical practices. They use their country presence and close partnerships with governments, international agencies, and/or nongovernmental partners to maximise scale up of the technologies for public health impact. J. Summary Value for Money Statement for the preferred option Criteria Economy – getting the best value from inputs Funding the PATH PDPs Management costs are included in the funding from DFID. The PDP works with and manages a wide range of partners across all stages of the pipeline, runs open competitions for innovations to include in the pipeline, All projects in the portfolio are subject to a detailed cost assessment by the PDP and its governance structures (scientific and technical advisory committees, Boards) at different milestoned stages along the development chain. Can leverage funding from private sector partners, work with developing country industrial partners and negotiate favourable costs and conditions to keep costs low. Efficiency maximising the outputs for a given level of inputs The PDPs are able to develop portfolios using the best candidates developed anywhere in the world. They make strong, rigorous decisions at predefined go/no go stages in the development chain and only progress candidates with a high degree of efficacy at each stage, thus decreasing risk of failure at each stage. Effectiveness ensuring that the outputs deliver the desired outcome PDPs act as neutral brokers, bringing together a wide range of partners, to work together. Some commercial partners would not be able to work together under other circumstances. Able to leverage in a wide range of in-kind support from different partners and have access to private sector and in-country development partners. Able to reach 32 PATH Product Development Business Case researchers, policy makers and regulatory authorities in developed and developing countries. Cost-effectiveness Acting as ‘virtual’ organisations PDPs use resources and knowledge from a wide range of partners. They are able to develop new technologies without the high overhead costs of maintaining complex infrastructure or a large workforce. 3 Commercial Case Delivery through a third party (multilateral organisation; civil society organisation) What assurance has been obtained on the organisation’s capability and capacity to deliver? Selection of the PDPs based at PATH was made following a global open competition. 47 organisations (including academic, industrial and public-private partnerships) submitted expressions of interest, 17 of which were invited to submit full proposals which all went through a formal process of scientific, technical, commercial, financial and policy peer review, discussions with other funders, private sector stakeholders, community stakeholders and researchers. A substantial number of staff employed by the PDP are from the private sector, including pharmaceutical and biotechnology industries and bring with them good knowledge and practices in the field. Core competences of organisation to deliver in country The PATH PDPs have a track record of working efficiently with numerous partners in many countries. Stringent portfolio management systems are employed which ensure that only the most promising leads are pursued. There are strict go/no-go decision points in the management of their portfolio of products which means that they are able to close out projects quickly that are unlikely to produce the desired outcome. The PDP has partnerships with organisations involved in the delivery of new technologies; to ensure that once developed they can be made available to those who need them. Procurement capability The organisation has demonstrated robust procurement systems and has a proven record in supply chain management and attaining economies of scale. Robust due diligence assessment has provided further assurance that procurement practices are undertaken professionally and in line with recognised best practice. PATH’s procurement processes and risk are regularly assessed by their own boards, and by funders, as part of the due diligence for providing support, by independent reviews of the organisation and members of the Multi Donor PDP Funders Group. Is there an opportunity to negotiate on the organisation’s anticipated costs? 33 PATH Product Development Business Case PDPs have been shown to deliver new products faster, and at lower cost, than the public or private sectors alone. They are able to leverage in resources, knowledge and expertise, from a range of different partners. The organisation will be constantly looking for potential savings that can be made during the term of the programme. The portfolio management enables the organisation to focus closely on products where there is a higher chance of success. The level of funding from DFID is less than that proposed in the organisation’s tender. Based on an analysis of the workplan, objectives and following discussions with other technical funders, our assessment is that there is an opportunity to invest up to £16 million in PATHs PDPs with increasing returns. DFID Relationship DFID has been a supporter of PDPs for some time, and based on previous experience and long term relationships with PDPs is viewed as a global leader in this area. DFID has worked with other funders, and PDPs themselves, to leverage support from other donors. The donor group (PDP Funders Group), currently chaired by DFID, has played a major role in influencing PDP governance, structure and financial planning. Donor input is welcomed by PDPs and donor harmonisation on reporting has created efficiencies. 4 Financial Case What are the costs, how are they profiled and how will you ensure accurate forecasting? The proposed budget for the PATH PDPs is £16,050,000 over 5 years (2013 –2018). The anticipated breakdown of core funding by DFID financial year is as follows but will be subject to negotiation with PATH based on need and avoiding payment in advance of need. Programme level costs Cost Categories Research costs Diarrhoeal disease drugs Diarrhoeal disease vaccines Malaria diagnostics Evaluation costs Total Research costs Overhead Total 5,220,000 5,220,000 780,000 780,000 6,000,000 6,000,000 3,480,000 50,000 520,000 4,000,000 50,000 13,970,000 2,080,000 16,050,000 34 PATH Product Development Business Case Amounts are £m except for the evaluation costs (exact timing of the evaluation to be agreed) Year 1 13/14 2 14/15 3 15/16 4 16/17 EDD OWH DxG 1.3 1.6 0.8 1.5 1.5 1.3 1.5 1.0 1.0 1.0 1.1 0.5 0.7 0.8 0.4 6 6 4 Total 3.7 4.3 3.5 2.6 1.9 16 Evaluation 5 Total 17/18 50,000 How will it be funded: capital /programme/admin? Funding will be made available from programme funds through a Memorandum of Understanding from the Human Development Team in Research and Evidence Division. How will funds be paid out? Support will be through a Memorandum of Understanding with PATH. Payments will be provided in June and November each year on production of appropriate claim forms. What is the assessment of financial risk and fraud? PATH will administer and account for DFID, and other funders’ contributions in accordance with its financial regulations and other applicable rules and procedures and practices. PATH has a proven record of strong financial management and probity employing international standards of financial management and accountability. The risk has been assessed as Low. Conditions will be included in the MOU to ensure that PATH have appropriate fraud and conflict of interest policies in place. How will expenditure be monitored, reported and accounted for? PATH will be required to submit Annual Audited Accounts for each of the years covered by the DFID grant. Harmonised annual reporting to donors will include detailed information on finances, in addition to technical, organisational and governance issues. 5 Management Case What are the management arrangements for implementing the intervention? The MOU will be managed by the Human Development Team in DFID’s Research and Evidence Division. They will have oversight through routine financial and performance reporting and review, including at regular donor meetings, when all donors will discuss the organisations scientific, technical, governance, organisational, operational and financial management. 35 PATH Product Development Business Case Within DFID a Health Adviser and Research Manager in the Human Development Team will provide the lead technical and project management roles, overseeing the relationship with PATH. A Deputy Programme Manager will be responsible for the day to day administration of the grant including financial tracking, compliance and other administrative functions. PATH is overseen by a Governing Management Board and there are Scientific Boards of experts for each individual PDP, all of which meet on a regular basis. What are the risks and how these will be managed? Overall the risk for this business case is HIGH. PATH and the individual PDPs undertake regular risk assessments, the Board has a role in the detailed scrutiny of risk. The following table provides a view of the major risks, technical, organisational and financial risks for the organisation which is reviewed internally throughout the year and will be reviewed annually by DFID at the time of the annual review. No Risk Technical risks Probability Impact Mitigation Measures 1 Generic Risk: PDP-associated risks are not adequately anticipated or managed. Medium High 2 Generic Risk: PATH-led PDPs may commit to advancing products that are not the most impactful and effective. Medium Medium PATH conducts formal risk assessment analyses for programs annually and prior to the start of new projects or partnership agreements. Risk assessments are carried out by product development teams with reviews by program and institutional leadership and are linked to the institutional review process for institutional strategic priorities, clinical trials and human subjects (when appropriate), financial benefits and impacts, and intellectual property. Risk avoidance, management, and mitigation strategies are included as core components of project planning and management activities and project/program reporting procedures. PATH has developed standard practices for active portfolio management both within and across its PDPs that include: Assessing target product profiles both internally and through external expert reviews. Establishing formal decision gates and go/no-go points throughout the product development process. Conducting evaluations before and after proof of concept as an essential components of the PDP decision-making process as well as a core element of evidence generation and dissemination of evidence to the broader scientific and public health communities. Conducting evaluations of global access as 36 PATH Product Development Business Case No Risk Probability 3 Generic Risk: The regulatory landscapes in targeted developing countries are not conducive for introduction or scale up of developed products. 4 Risk for G6PD High DxG: The quality of point-of-care G6PD tests will be unregulated and poor. Risk for G6PD Medium DxG: The companies developing products for target market may not have the commercial and technical capacity to deliver highquality products. Risk for vaccines Medium for diarrhea, ARVAC, EVI: Tropical gut enteropathy will reduce protective response for oral vaccines. Risk for vaccines Low for diarrhea, ARVAC, EVI: Inadequate funds and/or timelines for vaccine portfolio development. Risk for treatment Medium of pediatric diarrhea, DRG: Weak Pharmacovigilance infrastructure in many developing countries will limit the testing and risk 5 6 7 8 High Impact Medium Medium Medium Medium High High Mitigation Measures a core evidentiary base for PATH’s activities to facilitate scale-up and access to lifesaving technologies. PATH’s PDP target product profiles and go/no-go decisions include explicit consideration of introduction and regulatory contexts. PATH PDP and commercialization teams work with regulatory consultants early in the product development pipeline to identify strategies for registration. PATH partners with in-country ministries of health and academic institutions on clinical research to leverage partners’ existing relationships and experiences with regulatory approvals. The PATH-led PDP is working to establish evaluation guidelines and standards, as well as initiate discussion of viable and sustainable regulatory processes for the G6PD test. The PATH-led PDP has developed a series of tools for in-depth evaluation of the financial robustness of companies and the capacity of companies to take a product to market. The PATH-led PDP has also developed tools to verify the product development status and performance claims of companies. The PATH-led PDP has been investigating mucosal adjuvants, formulation and dose scheduling strategies, and parenteral vaccination. The PATH-led PDP has continued to seek funding from multiple sources and has developed contingency strategies for seeking out alternative developers and transferring IP, data, and regulatory responsibility. The PATH-led PDP continues to conduct training around pharmacovigilance in countries and regions in which we work. The PATH-led PDP integrates pharmacovigilance best practices into all clinical research as appropriate. 37 PATH Product Development Business Case No 9 1 1 2 Risk management associated with new products. Risk for treatment of pediatric diarrhea, DRG: Limited clinical trial resources in particular countries or regions (e.g., diarrhea research in Bangladesh) will limit ability of the PDP to test and introduce new products. Organizational risks PATH’s multiple and diverse partnerships may create the potential for conflicts of interest. Probability Impact Medium Medium Low High Financial risks Donor funds are not managed to high standards and levels of accountability. Donors’ funds are dispersed over multiple activities diluting impact. Low High Mitigation Measures The PATH-led PDP conducts training in Good Clinical Practice (GCP) for sites and administrators to raise the standard and understanding around clinical research principals and best practices. PATH works with partners to identify appropriate recruitment and enrollment strategies. PATH actively engages in technical capacity-building with in-country partners and sites. PATH maintains high institutional standards and clear strategies for creating and managing partnerships, described in the institutional publication Bringing New Health Technologies Within Reach for Everyone. PATH standards address issues of supply, intellectual property, and affordability of products and include clear procedures and standards for defining and managing potential conflicts of interest. PATH has adopted policies to be good stewards of funds we receive. We abide by institutional funders’ rules, regulations, and guidelines and we strive for the best stewardship of individual donors’ valued donations. We uphold applicable financial standards and principles in all aspects of our work. These include generally accepted accounting principles and standards defined by the Financial Accounting Standards Board, the International Accounting Standards Board, and the Governmental Accounting Standards Board. PATH maintains an internal audit team and undergoes annual external audits as core components of its financial management systems. PATH’s corporate and financial management systems have been designed with the flexibility to effectively and appropriately manage diverse sizes and scopes of donor funds. PATH uses active portfolio management strategies to allocate internal resources across technologies or projects, leverage contributions and available resources, and manage progress on product and institutional milestones. PATH’s management strategies are supported by project-specific financial and administrative managers and an institutional measurement system that tracks product development pipelines; project 38 PATH Product Development Business Case No Risk Probability Impact Mitigation Measures implementation milestones; and resource allocation across products, diseases, and institutional activities. What conditions apply (for financial aid only)? Not applicable How will progress and results be monitored, measured and evaluated? PATH has a detailed performance framework, which is used to manage performance internally and to demonstrate progress to the Board, Scientific Advisory Board and other funders. Internally all projects are monitored using strict go/no-go decisions and those that are not making their milestones are stopped. Evaluation DFID have allocated £50,000 for evaluation purposes. External reviews and/or evaluations are likely to be undertaken jointly with other donors. In addition it worth mentioning that the overall Board of Governors and the Science Advisory Board have a constant overview of the technical work being undertaken. Logframe Quest no. for draft logframe for PATH PDPs: 4025117. The programmes each have very detailed performance frameworks which they manage their performance internally and with their other donors. The Logframe is specific to DFID and will be discussed and agreed with the programme after DFID funding is agreed. 39