Palliative Care Services – Pharmacy Info leaflet
advertisement

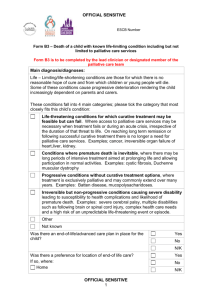
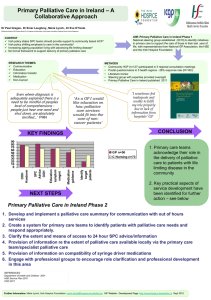
Pharmacy Information Sheet “A mixed methods investigation of community pharmacists’ delivery of palliative care services” You are being invited to take part in the above study looking at community pharmacists’ involvement in palliative care. Before you decide to take part it is important to understand why the research is being done and what it will involve. Please take time to read the following information carefully. Discuss it with your employer, other staff and relatives if you wish. Feel free to ask any questions if there is anything that is not clear or if you would like more information. Thank you for taking the time to read this leaflet. Palliative Care is defined by the World Health Organisation (WHO) as "…an approach that improves the quality of life of patients and their families facing the problem associated with lifethreatening illness, through the prevention and relief of suffering by means of early identification and impeccable assessment and treatment of pain and other problems, physical, psychosocial and spiritual” 1. About the study The study will combine quantitative and qualitative data (mixed methods) to investigate the delivery of palliative care services in community pharmacies. The aim of the study is to investigate availability and accessibility of palliative care medicines from pharmacies in Sheffield; identify the prevalence of errors on palliative care prescriptions; factors associated with timely access; and develop recommendations to inform providers and commissioners on improving access. There will be a data collection in pharmacies with some pharmacists chosen for interview on their experiences and perspectives in delivering palliative care services to inform the pharmacists’ role in the future. This will help us understand more about what helps and hinders pharmacists’ delivery of palliative care services and the processes by which patients and their carers obtain palliative care medicines. The study is expected to start in January 2016 and will be completed by January 2017. It is being completed as part of the researcher’s DPharm (professional doctorate) studies at the University of Bradford. 2. Why have I been chosen? You have been chosen because you are a pharmacist working in a Sheffield community pharmacy that either provides a commissioned service to palliative care patients and so will have experiences that will inform the research or because your pharmacy does not participate in such a service but can act as a comparator. We would like you to share your experience with us to help us explore the community pharmacists’ role in palliative care. 3. Do I have to take part? It is up to you to decide whether or not to take part. If you do decide to take part you will be given this information sheet to keep and be asked to sign a consent form. If you decide to take part you are still free to withdraw at any time without giving a reason. If you decide not to take part, you do not have to provide a reason and this will not affect your ability to participate in the provision of any locally commissioned services or future research projects. 4. What will it involve? The research will be conducted in 2 phases. If you decide to take part you will be asked in phase 1 to collect data on approximately 30 palliative care prescriptions presented to the pharmacy for 4 weeks over an 8 week period using a paper-based data collection form. It is likely that the data collection phase will be from the middle of January to March 2016 and you can decide which 4 weeks you will collect data. A payment of £100 will be paid to each pharmacy completing the data collection. There will also be a short customer survey linked to the data collection form. Data from the first phase will be analysed and in phase 2 five pharmacists will be selected and invited for an in-depth interview lasting between 45 and 60 minutes. Participation in the interview phase will again be optional. During this interview you will have an opportunity to discuss the results from phase 1, any issues that arose and answer further questions to understand your experiences and perspectives on delivering palliative care services. Interviews will take place in a convenient location often at your workplace at a mutually convenient time or can be arranged at St Luke’s Hospice. It is expected that interviews will take place between May and June 2016. A payment of £30 will be offered to interviewees. Participants can be kept updated electronically on the study’s progress and issued with a copy of the final report in February 2017. 5. What sort of questions will I be asked and will the information be recorded? If you are invited for interview you will be asked to share your experiences and views about palliative care with the researcher. For example, you may be asked what factors help or hinder the pharmacists’ input into palliative care, and what you see as the community pharmacists’ future role in palliative care. If you agree, we would also like to record some simple information such as number of years you have been registered, training and experience. The information you provide will be audio recorded with your consent. The researcher may also take notes of the interview but will not record any identifiable information. All information disclosed will be treated with the strictest confidentiality and the audio recording will only be shared with a medical secretary for the purpose of transcribing the interview. Any information that could identify you will be removed by the researcher during the transcription. You will be given the opportunity to check the interview transcript to ensure you are happy with this. 6. What information am I being asked to collect in phase 1? You will need to collect data on approximately 30 palliative care patient prescription forms over a period of 4 weeks. This will include recording the time from when a prescription is presented to the pharmacy to when it is ready for collection, information on the drugs prescribed, whether there were any errors on the prescription, the type of error, and any interventions. A copy of the data collection form is available on request. No patient identifiable information is collected. 7. What if something goes wrong? There are no specific risks associated with taking part in this study and it is highly unlikely that you will be harmed or compromised in any way. However, if you have any cause to complain about any aspect of your treatment while you are taking part in the study, or any other concerns please contact the project J.D.Morgan1@bradford.ac.uk supervisor, Dr Julie Morgan, on 01274 234766 or 8. What are the possible benefits of taking part? You will contribute to improving local services in Sheffield for palliative care patients and promote the role of the community pharmacist within this to local commissioners. You will have the opportunity to participate in research as part of your continuing professional development and use the results to improve services in your pharmacy. This may also provide you with CPD (Continuing Professional Development) opportunities. 9. What will happen to the results of the research study? Findings from the study will be reported to the Sheffield LPC and commissioners to support improving services for patients. They will also be shared across local and professional networks in pharmacy and palliative care. It is expected that results will be reported in poster and abstract form at national pharmacy and palliative care conferences as well as in academic journals. Any information or advice that you share will be anonymised in such a way that it cannot be attributable to any individual or employer. The information you provide will not be used if you decide that you do not want your information to be used in the study. 10. Will I see the results of the study? You may request a copy of the study findings which will be sent to you following completion. 11. Who is organising and funding the research? The research is sponsored by the University of Bradford with supervision by Dr Julie Morgan and Professor Alison Blenkinsopp. Support and funding is provided by Sheffield Teaching Hospitals Trust, St Luke’s Hospice and a personal award from Pharmacy Research UK. 12. Who has approved the study? Ethical approval has been granted by the Chair of the Biomedical, Natural, Physical and Health Sciences Research Ethics Panel at the University of Bradford on 17th December 2015. Sheffield Teaching Hospitals NHS Trust and Sheffield Research Consortium on behalf of the Sheffield Clinical Commissioning Group have confirmed no further approvals are required. 13. What happens next? If you are interested in taking part please contact the researcher to discuss further. You will then be given time to decide on whether you wish to take part. If you do agree to take part you can withdraw your consent at any time without giving a reason. 14. Contacts for further information To participate in the study, or if you would like further information please contact Elizabeth Miller, Palliative Care Pharmacist on 0114 236 9911 (Tues to Fri) or 07957 160642 or via E.J.Miller1@student.bradford.ac.uk Thank you for reading this information Elizabeth Miller, Registered Pharmacist Date: 28th October 2015 Version number 3 Consent form to Participants (Phase 1) (To be sent electronically to all pharmacists participating in the data collection in phase 1. One copy unsigned for the participant and signed copy to be kept by the researcher.) My name is Elizabeth Miller. I am doing a research study investigating the community pharmacists’ delivery of palliative care services in Sheffield. The project is being completed as part of my DPharm professional doctorate studies at the University of Bradford. I am directing the project and can be contacted on 0114 236 9911 (9-5pm Tuesday to Friday) or 07957 160642 or on E.J.Miller1@student.bradford.ac.uk should you have any questions. Thank you for agreeing to take part in the project. Before we start I would like to emphasize that: - You participation is entirely voluntary; You are free to refuse to answer any questions; You are free to withdraw at any time. I understand that the data I collect and any information I provide will be kept strictly confidential and will only be available to members of the research team. Please sign this form to show you agree with the above. ___________________________________________________ (signed) ___________________________________________________ (printed) _________________ Date Please send a report on the results of the project: YES NO (circle one) Address for those requesting a research report ___________________________________________________ ___________________________________________________ One copy to be left with the participant and one copy to be signed and kept by the researcher