Name:
advertisement

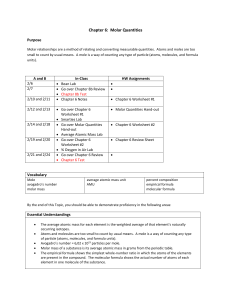
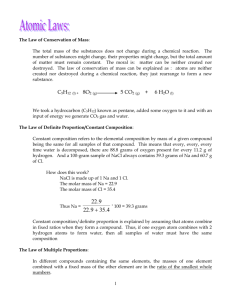
Name: Date: 5.4 Topic: Balancing Chemical Equations Learning Objectives: 1. I can explain why a chemical equation needs to be balanced according to the Law of Conservation of Mass 2. I can balance a chemical equation 3. I can determine the molar ratio between two chemicals from a balanced chemical equation ________________________________________________________________________________ Bellringer 1. What is the Law of Conservation of Mass? 2. For the chemical reaction below, fill in the missing mass: LiCl + MgS Li2S + MgCl2 7g + 3g 4g + ____g 3. What does the word “accountability” mean to you: Week of 11/11-11/15: ACTION PLAN What is my current average in this class (Look at your character report card): _____________ Am I missing any assignments/tests in this class? (Am I missing the Unit 4 Test?) _____________ OR… Am I planning on RETAKING the Unit 4 Test? _______________ If your answer was yes to either… WHEN do you plan on retaking the test? (Check one): ☐ Other ☐ : __________________________________________ Thursday at Lunch Have I been ABSENT for any days of this class during this unit? 5.1: Physical and Chemical Changes (Tues) : __________ (yes or no) 5.2: Types of Reactions (Thurs) : ___________ (yes or no) If your answer was yes… What is your plan for catching up? (Check one): Get the notes online ☐ Come to the review session on Thursday ☐ On a scale from 1 to 5, how comfortable am I with the material in this unit (5 = I understand everything, 1 = I have no idea what is going on) CIRCLE ONE 1 2 3 4 5 If your answer was 3 or less, how do you plan to review the material before the test? Take notes home to study ☐ Come to the review session on Thursday ☐ This week’s action Items: What do I need to do? When do I need to do it? How will I remind myself? Notes: KP 1: What does the Law of Conservation of Mass say? Matter can neither be created or destroyed Every element has to have the same number of ____________ on the left (reactants) side and right (products) side of a chemical equation. When this is true, the chemical equation is “__________________” Example: o NOT Balanced, cannot occur in nature: N2 + O2 NO2 (N atoms appear to be destroyed) o Balanced, can occur in nature: N2 + 2O2 2NO2 (N atoms and O atoms are conserved) o If you have ___ Nitrogens on the left side, you have to have ____ Nitrogens on the right side because Nitrogen atoms can neither be created nor destroyed in a chemical reaction! KP 2: How do I count atoms? #Atoms = Coefficient x Subscript o Coefficient =Big Number in front (applies to the WHOLE compound!) o Subscript = Little Number on bottom right (applies only to the element before it) o No number means “1” Example #1: 2SO3 o There are 2 Sulfur atoms because 2x1=2 o There are 6 Oxygen atoms because 2x3=6 Example #2: Li3N o There are ____ Lithium atoms because _____________ o There is _____Nitrogen atom because ______________ Example #3: 4C2H3O2 o There are ______ Carbon atoms because _____________ o There are ______ Hydrogen atoms because _____________ o There are _______Oxygen atoms because ______________ Practice Counting Atoms 1. NaOH Na: ____ H:_____ O: ____ 2. 3CH4 3. 6H2S C: ____ H: ____ H:____ S: ______ 4. Mg2SO4 ______ Mg: ____ S: _____ O: 5. 2SO3 S: _____ O:________ KP 3: How can I balance a chemical equation? 1. BOX: Draw ___________ around each chemical formula 2. COUNT: Write how many atoms of each element are on the _______ and ____________ COEFFICIENT: Pick one __________________ element to start with. On the side that has LESS, put a BIG number to make both sides equal. Continue until all elements are balanced! 3. Leave ________________ and _______________ for last! 4. NEVER change anything in the boxes…just add coefficients! Examples of Balancing Chemical Equations: 1. _____ H2 + _____ O2 _____ H2O 4. __CH4 + __O2 __CO2 +__H2O 2. _____ N2 +_____ H2 _____ NH3 5. _____ N2 + _____ O2 _____ N2O 3. _____ S8 + _____ O2 _____ SO3 6. _____ HgO _____ Hg + _____ O2 Key Point 4: How do I determine the molar ratio between two chemicals? The molar ratio is the ratio (______________) of the _______________________ between 2 chemicals in a balanced chemical equation To find the molar ratio between 2 chemicals, first balance the equation, and then write the coefficients for the chemicals as a ratio or fraction Example: 4NH3 + 5O2 4NO + 6H2O 1) What is the molar ratio between NH3 and NO? _________ 2) What is the molar ratio between NH3 and H2O? __________ 3) What is the molar ratio between O2 and NO? ___________ 4) What is the molar ratio between O2 and H2O? ___________ Independent Practice: Macro (BIG) Explain why all chemical equations must be balanced. Nano (small) Symbolic NO2 + 2Li2Se NSe2 + 2Li2O Using the equation above, what is the correct molar ratio between NO2 and NeS2? C + H2 CH4 Explain why this chemical equation can never occur (Hint: look back at Key Point #1) What is the correct molar ratio between Li2Se and Li2O? What is the correct molar ratio between NO2 and Li2O? Balance the following equations so that they follow the Law of Conservation of Mass. 1) ___Li + ___CuCl2 ___LiCl + ___Cu 2) ___Fe + ___S8 ___FeS 3) ___C3H8 + ___O2 ___H2O + ___CO2 4) ___Al + ___O2 ___Al2O3 Explain how you know that the following chemical equation could not occur. Be + Cl2 Be2Cl3 ________________________________________________________________________________ ________________________________________________________________________________ Which of the following equations best illustrates the Law of Conservation of Mass? Why? 1) C2H3 + O2 CO2 + H2O 2) 2Li + MgF2 2LiF + Mg 3) Ca + F2 2CaF2 ________________________________________________________________________________ ________________________________________________________________________________ Use the following equation to answer the questions below: Be + F2 Be2F3 a. What is wrong with the equation? b. Fix the equation by writing it correctly in the space below.