Name: Date: Covalent Bonding Heat Retention Graphing Lab
advertisement

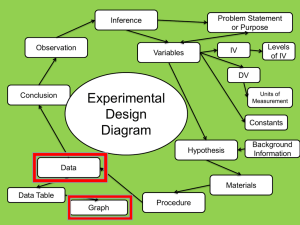
Name:__________________________________ Date:______________ Covalent Bonding Heat Retention Graphing Lab Background: In class, you have learned that covalent bonds have low boiling points, low melting points, and that they are brittle when in the solid state. Mr. Haidinger placed water in a pot and peanut oil in another pot. He heated both on his stove at the same temperature for 10 minutes. Below you will find the data for the temperatures of both water and oil. You will use this data to create a formal analysis and conclusion section of a lab write up. Molecular Structures *Water = H20 Peanut Oil = CH3(CH2)7CH Data Time (min.) 0.0 0.5 1.0 1.5 2.0 2.5 3.0 3.5 4.0 4.5 5.0 5.5 6.0 6.5 7.0 7.5 8.0 8.5 9.0 9.5 10.0 Water Temperature (°F) 61 63 79 81 97 100 110 119 120 130 139 147 150 160 161 178 180 180 180 180 182 Peanut Oil Temperature (°F) 61 63 80 100 120 140 170 200 220 245 275 300 330 360 375 415 435 450 475 485 525 Graph: Create ONE line graph of the data using graph paper and a ruler. Place time on the x-axis and temperature on the y-axis. Time is the independent variable and temperature is the dependent variable. Dependent variables are always placed on the x-axis and independent variables are always placed on the y-axis. Analysis and Conclusion Create an analysis and a conclusion section based on the data and your graph. Use the guidelines from “How to Write a Formal Lab Write Up” to help you complete these sections.