Copper Cycle Lab Instructions
advertisement
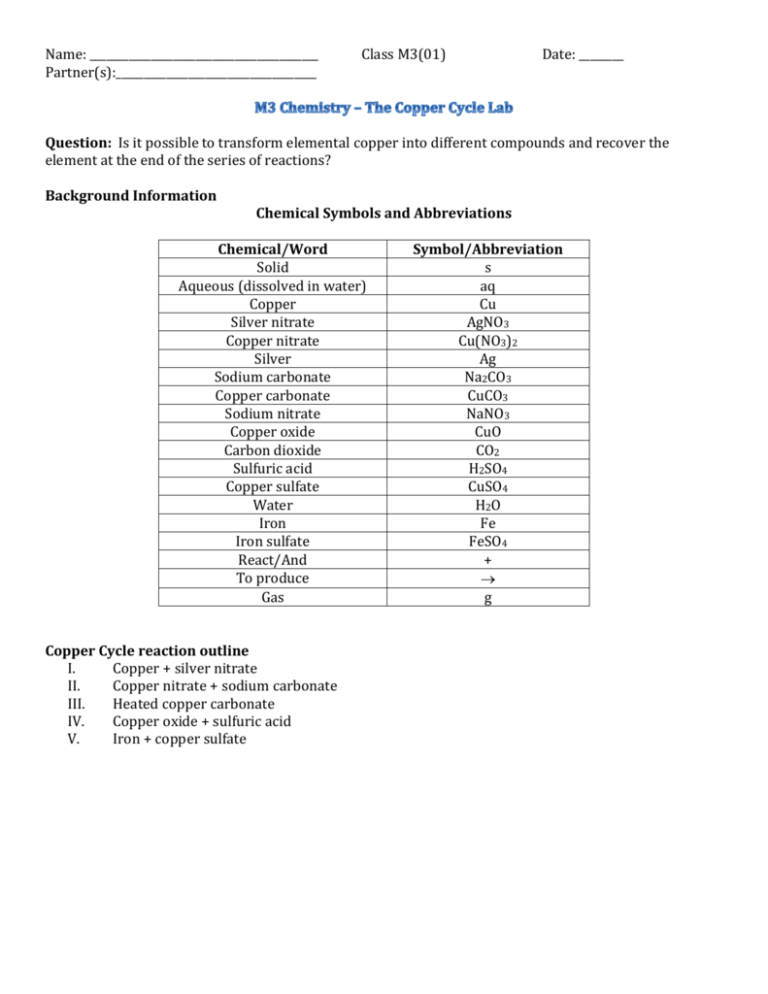
Name: _________________________________________ Partner(s):____________________________________ Class M3(01) Date: ________ Question: Is it possible to transform elemental copper into different compounds and recover the element at the end of the series of reactions? Background Information Chemical Symbols and Abbreviations Chemical/Word Solid Aqueous (dissolved in water) Copper Silver nitrate Copper nitrate Silver Sodium carbonate Copper carbonate Sodium nitrate Copper oxide Carbon dioxide Sulfuric acid Copper sulfate Water Iron Iron sulfate React/And To produce Gas Copper Cycle reaction outline I. Copper + silver nitrate II. Copper nitrate + sodium carbonate III. Heated copper carbonate IV. Copper oxide + sulfuric acid V. Iron + copper sulfate Symbol/Abbreviation s aq Cu AgNO3 Cu(NO3)2 Ag Na2CO3 CuCO3 NaNO3 CuO CO2 H2SO4 CuSO4 H2O Fe FeSO4 + g Part I: Copper + silver nitrate Materials: Copper wire, silver nitrate solution, 2 test tubes, test tube rack, magnifying glass, lamp Procedure: 1. DOCUMENT EACH STEP OF THE PROCEDURE WITH EITHER PHOTOS OR VIDEO. 2. Place 5 mL (2.5 cm) of silver nitrate solution into a test tube. 3. Add a small piece of copper wire, bending the top of the wire to act as a handle above the test tube. 4. Observe the results over time using the magnifying glass and lamp. 5. Video or photograph the changes over time. Connect written observations to the media, either as titles, captions or call-outs (like the speech bubbles in comic books). 6. After about 10 minutes decant the solution through a filter paper into a clean test tube. 7. Save the decanted solution for Part II. Be sure to label with your team’s names. Word equation for this reaction: Copper (s) reacts with silver nitrate (aq) to produce copper nitrate (aq) and silver (s) Use the table above to write the chemical equation with the correct symbols: Put this equation and all your documentation and observations in your eLab report. Part II: Copper nitrate + sodium carbonate Materials: Copper nitrate solution (from Part I), sodium carbonate solution, 2 test tubes, 50 mL beaker Procedure: 1. DOCUMENT EACH STEP OF THE PROCEDURE WITH EITHER PHOTOS OR VIDEO. 2. Place about 3 mL of sodium carbonate solution in a clean test tube. 3. Add the copper nitrate solution from Part I to the test tube containing sodium carbonate solution. 4. Document and record your observations. 5. Filter the precipitate using the apparatus shown by your teacher. 6. After filtration, place the filter paper and residue to dry. 7. Create a DIAGRAM of the filtration apparatus. 8. Save this dried material for Part III. Be sure to label with your team’s names. Word equation: Copper nitrate (aq) reacts with sodium carbonate (aq) to produce copper carbonate (s) and __________ Chemical equation: Put this equation and all your documentation and observations in your eLab report. Part III: Heating copper carbonate Materials: Flame source (Bunsen burner, e.g.), matches, Pyrex test tube, wooden tongs, safety glasses, dry copper carbonate (from Part II) Procedure: 1. DOCUMENT EACH STEP OF THE PROCEDURE WITH EITHER PHOTOS OR VIDEO. 2. Place the dry copper carbonate (from Part II) into a test tube. 3. Using the tongs, heat the test tube in the Bunsen burner’s flame for a few minutes. BE EXTREMELY CAREFUL AND TAKE THE NECESSARY SAFETY PRECAUTIONS 4. Save the resulting material for Part IV. Be sure to label with your team’s names. Word equation: Copper carbonate (s) is heated to produce copper oxide (s) and carbon dioxide (g). Chemical equation: Extension question: How could you test to see if the gas was carbon dioxide? Put the answer to the extension question, the chemical equation, and all your documentation and observations in your eLab report. Part IV: Copper oxide + sulfuric acid Materials: Copper oxide (from Part III), 1 M sulfuric acid, dropper, glass rod Procedure: 1. DOCUMENT EACH STEP OF THE PROCEDURE WITH EITHER PHOTOS OR VIDEO. 2. Let the test tube containing copper oxide from Part III cool down for 5 minutes. 3. Add 4 mL of sulfuric acid CAUTION: SULFURIC ACID IS VERY CORROSIVE!!! 4. Record your observations in your eLab report. 5. Save the solution for Part V. Be sure to label with your team’s names. Word equation [one product of the reaction is copper sulfate(aq)]: Chemical equation: Put the chemical equation and all your documentation and observations in your eLab report. Part V: Iron + copper sulfate Materials: iron nail, string, copper sulfate solution (from Part IV) Procedure: 1. DOCUMENT EACH STEP OF THE PROCEDURE WITH EITHER PHOTOS OR VIDEO. 2. Attach a piece of string to an iron nail. 3. Suspend the iron nail in the solution of copper sulfate (from Part IV). 4. Wait. 5. Record your observations. 6. Save the resulting materials for proper disposal. Word equation: Chemical equation: Put the chemical equation and all your documentation and observations in your eLab report. OVERALL CONCLUSION based on all five experiments. As a team and using the document “How To Write A Conclusion” from mshorr.weebly.com, create an overall conclusion and include it in your eLab report. To make your conclusion the best, be ready to do a few drafts and proofread it. Writing is rewriting.





