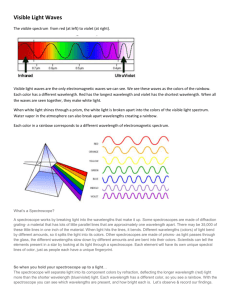
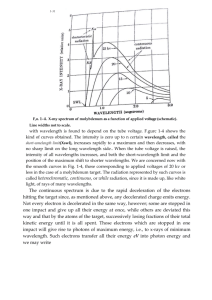
Briana Byrne 5th hr Spectrum Lab Intro: Everyday, doctors are
advertisement

Briana Byrne 5th hr Spectrum Lab Intro: Everyday, doctors are treating new and different things. Behind every disease or problem a patient may come along with is the origin of past symptoms a doctor has observed. Doctors make decisions on what to do based on what they have learned and what they have experienced/done in the past. Based on what was observed during this lab and what was recorded from the spectroscope, the unknowns should be easy to figure out. Wavelengths are recorded by the calibrated scale on the spectroscope designed to show the wavelength of each color of the spectrum. A line spectrum is how the wavelengths are represented in a spectroscope. This means that the color spectrum will have distinct lines at a specific wavelength for that color. To determine the percent error, the equation (true-experimental)/true is used. Comparing the calculated wavelength and the actual length is how this will be found, determining whether the spectroscopes were calibrated correctly and if the lab was accurate. If the wavelengths of emission lines are determined then the unknowns may be determined. Materials & Methods: Each element was poured into a Petri dish and lit. Then, the flame was observed through a spectroscope and each color was recorded with the number at which the light appeared. Results: Table 1: Spectrum tube Hydrogen Observed color purple blue teal Lime green Yellow Orange Red Observed wavelength 440 nm 460 nm 500 nm 550 nm 590 nm 620 nm 650 nm True wavelength % error Light: 410 nm Dark: 435 nm Light: 7% Dark: 1% 660 nm 1.5% Briana Byrne 5th hr Lithium Strontium Potassium Color Purple Dark green Yellow green Lime green Yellow Orange Red Observed TRUE %error 450 nm Not listed 500 nm 550 nm 530 nm 600 nm 640 nm 700 nm Color Observed Purple TRUE %error 450 nm 405 nm 11% Blue 510 nm 445 nm 14% Blue green 570 nm 500 nm 14% Orange 610 nm 585 nm 4% Red 660 nm 670 nm 1% Color Observed TRUE %error Purple 440 nm 405 nm 8% teal 500 nm 480 nm 4% lime green 570 nm 535 nm 6.50% orange 620 nm 610 nm 1.60% red 640 nm 695 nm 7.90% Color Observed TRUE %error Briana Byrne 5th hr Sodium Calcium Barium purple 450 nm 440 nm 2.30% teal 500 nm 495 nm 1% green 580 nm 520 nm 11.50% orange 620 nm 590 nm 5% red 640 nm Color Observed TRUE %error dark purple 400 nm 390 nm 1% light purple 440 nm 420 nm 4.70% teal 500 nm 500 nm 0% lime green 570 nm 530 nm 7.50% orange 610 nm 620 nm 1.60% red 660 nm 645 nm 2.30% Color Observed TRUE %error purple 420 nm 415 nm 1.20% teal 460 nm 495 nm 7% lime green 580 nm 555 nm 4.50% orange 630 nm 615 nm 2.40% red 670 nm 650 nm 3% Color Observed TRUE %error Briana Byrne 5th hr Unknown1 purple teal 520 nm green 560 nm lime green 590 nm orange 620 nm red 710 nm Color Observed Unknown2 purple Light 450 nm 450 nm unknown TRUE %error unknown teal 520 nm green 560 nm orange 620 nm red 710 nm Color Observed Purple 430 nm 400 nm 7.5% blue 450 nm 450 nm 0% teal 500 nm 490 nm 2% green 560 nm 520 nm 7.7% yellow 610 nm 590 nm 3.4% orange 640 nm 620 nm 3.2% TRUE %error Briana Byrne 5th hr Ethanol Color Observed Purple 450 nm teal 500 nm green 560 nm orange 640 nm TRUE %error Not listed Table 2: Flame spectrum wavelength related to energy transitions Level 6 to 2 : 2.19 * 1023 level 5 to 2 : 2.07 * 1023 level 4 to 2 : 1.85 * 1023 level 3 to 2 : 1.37 * 1023 Sample calculation of a wavelength: 1/lambda = RH/hc (1/nf2 – 1/ni2) Level 6 to 2: 1/lambda = RH/hc (1/22- 1/62) = 2.19 * 1023 Conclusion: The lab results made sense and were very close to the true wavelength. All spectroscopes were calibrated the same. The amount of light in the room affected the spectrum because some of the light was included in the calculations, therefore throwing the numbers off a little. But, in order to see the scale it was necessary to have light in the background while observing. I would need line spectrum measurements to determine for sure the unknown in order to compare exact numbers. But, the unknowns assumed to be in the first were Barium and potassium; in the second unknown, potassium and strontium. In the first unknown, barium matches or is close to most of the colors calibrated in the lab and potassium is almost exactly the same numbers of wavelength as the unknown. The second unknown was determined to have potassium because the wavelengths match very close, and strontium is also very close in the number wavelengths for each color in the spectrum. There was little percent error, but, where the error occurred was from the background light or the angle the flame was being observed. Mechanics need to know what Briana Byrne 5th hr past vehicles have had wrong with them and observe what could be going wrong in order to find the problem. They have a mind trained to listen for certain things from experience in order to be successful. This lab uses past wavelengths to compare to the unknowns in order to figure them out.