Supplementary Information (docx 747K)
advertisement

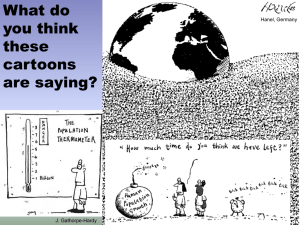
1 2 3 4 5 6 7 8 9 10 11 12 13 Supplementary Information - Lineage key Figures SI Figure 1 to SI Figure 6 Tables SI T1 to SI T3 Detailed methods SI reference list Lineage key: Lineage key used throughout SI. 14 15 Figures Oxygen evolution Oxygen consumption 1.50 Fold change to trait value in selection environment before CO2 enrichment 2.0 1.25 1.5 1.00 1.0 0.75 0.5 0.50 before after Chlorophyll content 1.2 before after Size 1.4 1.1 1.2 1.0 1.0 0.9 1.50 before after Lipid content before after Selection environment 1.25 SA FA 1.00 0.75 0.50 before 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 after Figure 1A: The plastic responses of SA and FA evolved lineages to elevated CO 2 levels are reversible before (left) and after (right) short term exposure to elevated pCO 2. After 7-14 generations at 1000µatm CO2, lineages were placed back in their respective environment, and a subset of phenotypic traits was measured after two transfers (7-14 generations). Importantly, growth rates were not impaired when lineages from either selection regimes were grown at 430µatm after short term CO2 exposure ( Schaum & Collins, 2014). Likewise, there were no significant differences between traits before and after short-term exposure to elevated CO2 levels. (ANOVA stability x before/after F1,301 =0.2542 , p= 0.6145). SA in purple, FA in blue. All values are displayed as fold changes compared to SA evolved lineages or FA evolved lineages prior to short term exposure to elevated CO 2 , depending on the selection environment lineages were evolved in. N=3 per lineage. Number of lineages = 16. Dotted line indicates fold change 1 compared to lineages prior to short-term exposure to CO2 for SA and FA respectively (i.e. no change). Units that fold changes were calculated from are: Oxygen evolution and consumption in fmol/cell*hour. Chlorophyll content in fg/cell. Size as diameter in µm. Lipid content is for polar lipids calculated from Nile Red Fluorescence units and in fg/cell. 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 SI Figure 1 B: Phenotypes of correlated responses of SH and FH evolved lineages. SH displayed as purple, FH as blue. When SH and FH evolved lineages were assayed at 430µatm CO2, growth rates were greatly reduced in SH evolved lineages, but not in FH evolved lineages ( Schaum & Collins, 2014). Of the SH lineages that did grow (albeit very slowly), oxygen evolution was reduced (Tukey post-hoc on omnibus ANOVA testing for differences between SH and FH, p<0.05), but oxygen consumption was not, which may partially explain the impeded growth rates. Size and chlorophyll content did not differ significantly between SH and FH lineages, and were also not significantly different from FA (for FH evolved lineages) or SA (for SH evolved lineagse). Note that SH lineages assayed at 430 ppm were growing so poorly that no C:N samples could be obtained. Nile Red Fluorescence was below the detection limit in SH and FH evolved lineages when assayed at 430µatm CO 2. Units that fold changes were calculated from are as in SI Figure 1A. 47 48 49 50 51 52 53 54 55 56 SI Figure 2: Size selective gating of chlorophyll positive events. Side Scatter (SSC – proxy for granularity) and Forward Scatter (FSC – proxy for size) gating of chlorophyll a positive cells (orange) and chlorophyll negative cells (red) on a FACS CANTO. The SSC/FSC gate was established using calibration beads of different sizes. The same gates were used to distinguish bacteria from algae in our samples. A SYBR green stain had been used beforehand in order to distinguish debris from DNApositive particles, as Ostreococcus, with cells as small as 1µm is often in the same size range as debris. 57 58 59 60 61 62 63 64 65 66 67 68 69 70 71 72 73 74 75 SI Figure 3: In SH and FH evolved lineages the phenotype characteristic for plastic response of SA and FA evolved lineages is not maintained in the long-term, i.e. the plastic response gradually reverses for many traits. For t0, t100, and t400, we display fold changes of mean trait values in FH evolved lineages compared to FA evolved lineages, and fold changes of mean trait values in SH evolved lineages compared to SA evolved lineages – the dotted line indicates a fold change of 1, i.e. no change. Traits are colour coded and represented by individual symbols (see legend). After 75-100 generations of evolution in SH or FH, lineages begin to show deviations from the plastic response (compare to SI Figure 1A, where the plastic response is reversed almost completely after 7-14 generations) with significant differences between SH and FH evolved lineages for individual traits (ANOVA F5,138 = 5.3269 , p<0.01). At t400 (400 generations into the experiment), mean trait values are different between FH and SH, and differ from the plastic response as discussed in the main text. 76 77 78 79 80 81 82 83 84 85 86 87 88 89 90 91 92 93 94 95 96 97 SI Figure 4: In the ambient or plastic response, there are lineage-specific differences between SA and FA evolved lineages. Likewise, lineages vary in their phenotypic evolution to SH or FH. (ANOVA (all traits) F15 ,138 = 2.51, p<0.05) ) Mean trait values per lineage are represented by individual symbols (see key). Error bars omitted for clarity (but see SI Figure 6, where lineages have been pooled according to sampling depth). Blue slopes are linear models fitted for each pair of lineages in each environment. Differences between lineages from stable and fluctuating environments are depicted for the ambient response (SA and FA evolved lineages assayed at 430µatm CO 2), the plastic response (SA and FA evolved lineages assayed at 1000µatm CO2), and the evolved response (SH and FH evolved lineages assayed at 1000µatm CO2). Differences between lineages across selection regimes are shown as differences in direction or steepness of slope. For example, oxygen evolution rates are on average not different between SH and FH evolved lineages (see main text for statistics). On the level of the individual lineage, however, there are significant differences (graphic representation direction of slope), with rcc809, rcc789, rcc675, rcc501, rcc1662, rcc1645 and rcc1558 having higher oxygen evolution rates after evolution in FH than after evolution in SH (all post hoc p<0.05), while rcc422, rcc410, rcc343, rcc1114 and rcc1108 have significantly lower oxygen evolution rates in FH than in SH (all post hoc p <0.05). All lineages have lower C:N ratios after evolution in FH than after evolution in SH, but the amount by which C:N is lower (graphical representation: steepness of slope) differs between lineages. 98 99 100 101 102 103 104 105 106 107 108 109 110 111 SI Figure 5: Since the phenotypic changes of SH and FH are not well correlated with each other for most traits, evolutionary responses in SH cannot be used to predict evolutionary responses in FH. For lineage legend see beginning of SI (or main manuscript Figure 3). Lineages are displayed as means for 3 biological replicates; error bars are omitted for clarity. The blue line is a linear model fitted to the means. The r2 value is only significant for Nile Red Fluorescence, but this might be an artifact of lineages displaying either no or high Nile Red fluorescence after evolution in SH or FH. The units that fold changes were calculated from are as in SI Figure 1. 112 6 8 12 6 9 Lipid Size Chlorophyll content C:N ratio R:P ratio Oxygen consumption Oxygen evolution 12 0.6 2.0 10 4 8 1.5 Trait Value 0.4 Isolation depth 4 surface 6 10-40 metres >100 metres 1.0 5 2 4 0.2 2 3 0.5 113 114 115 116 117 118 119 120 121 122 123 124 SH FH SH FH SH FH SH FH 0 0.0 0 0 0.0 0 0 SH FH SH FH SH FH Selection environment SI Figure 6: There are significant within and between selection regime variations in evolved trait values depending on where lineages of Ostreococcus had been sampled from originally (ANOVA (all traits) F 2,153 = 3.88, p <0.05) Isolation depths are colour coded with red = sampled at the surface, green = sampled at 10-40 metres, blue = sampled at >100 metres. Bars are for mean trait values ± 1 SD. Oxygen evolution and consumption are in fmol/cell*hour. Chlorophyll content is in fg/cell. Size is the average diameter in µm. Lipid content is for polar lipids calculated from Nile Red Fluorescence units and in fg/cell. 0.4 PC2 0.2 0.0 SH evolved FH evolved SA evolved FA evolved Large symbols: isolated 0-60 metres Small symbols: isolated >60 metres -0.2 -0.2 125 126 127 128 129 130 131 132 133 134 135 136 137 0.0 0.2 0.4 PC1 SI Figure 7: Colour-coded version of Figure 3. Both environmental stability (PC1) and CO 2 elevation (PC2) drive phenotypic evolution of lineages. Large symbols lineages sampled from above 60 meters depth, small symbols: lineages sampled from below 60 meters depth. Red: SH evolved lineages, blue: FH evolved lineages, black: SA evolved lineages, green: FA evolved lineages. Each lineage is represented by a unique symbol. Together, fluctuation and elevation of pCO2 explain about 90% of the variance in evolved phenotypes, and lineages (within CO 2 treatments) cluster largely according to sampling depth. 138 139 140 141 142 143 144 145 146 TABLES SI Table 1: Sampling locations of Ostreococcus lineages used in this experiment. We obtained oth95, rcc809 and rccc501 from the Plymouth Marine Laboratory (UK). All other strains were supplied from the Roscoff Culture Collection through an ASSEMBLE grant. More information on the strains used is available at http://www.sbroscoff.fr/Phyto/RCC/index.php?option=com_dbquery&Itemid=34. We present a more detailed table as part of the SI to ( Schaum et al., 2013), but are also making the information on sampling stations and sampling depth available here for easier access. 147 Lineage 148 149 Latitude Longitude Sampling station Depth First [m] isolated oth95 43º 24' 3º 36' Thau Lagoon 0m 1996 rcc1107 43º 3' 2º 59' Mediterranean 0m 2006 rcc1108 42º 29' 3º 8' Mediterranean 0m 2006 rcc1114 42º 48' 3º 1' Mediterranean 0m 2005 rcc1558 42º 48' 3º 1' Mediterranean 0m 2005 rcc1645 48º 46' 3º 56' North Sea 10m 2007 rcc1662 50º 12' 0º19' English Channel 10m 1995 rcc434 30º8’ 10º3’ Atlantic Ocean 40m 1999 rcc410 29º 28' 34º 55' Red Sea 100m 2000 rcc422 48º 37' 3º 51' English Channel 0m 2001 rcc501 48º 46' 3º 1' Mediterranean 10m 1995 rcc675 54º 11' 7º 54' North Sea 0m 2001 rcc747 42º 35' 8º 49' Atlantic Ocean 10m 1995 rcc789 not known not known Spanish Coast 0m 2001 rcc809 not known not known West Mediterranean 105m 1995 rcc810 21º 2' 31º 8' Atlantic Ocean 120m 1991 150 151 152 153 154 155 156 157 158 159 160 161 162 SI Table 2: Carbonate chemistry table Note that we also present this data as part of the SI to (Schaum & Collins, 2014). However, for easier access, we are also including it in this paper. Carbonate Chemistry: pH was measured routinely for all samples; DIC was measured for a random at each transfer, and for all samples at the beginning and the end of the selection experiment. Data for each time point in each selection regime was calculated from a minimum of six samples. Total Alkalinity (TA) was measured at the beginning, 100 generations into the experiment and at the end of the selection experiment. All other values were calculated using seacarb within R for 18 ºC and salinity 32. pH was determined for each sample at the end of each transfer. As samples were dilute with maximum densities not exceeding ~ 5*104 cells per ml, pH did not drift more than 0.05 units. Treatment Control HCO3- CO32- DIC TA [µmol kg-1] [µmol kg-1] [µmol kg-1] [µmol kg-1] 8.001 1990.48 154.88 2161.94 2369.52 426.43 8.030 1900.95 158.09 2073.84 2290.35 430 466.76 8.013 1999.06 159.72 2174.99 2389.33 4 430 478.44 8.003 2003.79 156.56 2176.96 2386.41 5 430 477.79 8.003 2001.57 156.42 2174.59 2383.95 6 430 431.15 8.021 1880.38 152.99 2048.35 2258.28 7 430 367.72 8.101 1929.99 188.97 2131.74 2391.65 8 430 462.38 8.016 1996.29 160.78 2173.14 2389.16 9 430 455.04 8.023 1993.73 162.96 2172.50 2391.85 10 430 339.35 8.130 1904.46 199.39 2115.64 2391.56 11 430 455.43 8.022 1993.62 162.80 2172.24 2391.36 12 430 373.96 8.029 1664.34 138.19 1815.52 2013.85 13 430 343.32 8.111 1843.84 184.73 2040.49 2298.58 14 430 475.92 8.000 1980.08 153.69 2150.30 2356.58 15 430 484.24 8.001 2020.15 157.22 2194.19 2403.88 16 430 343.91 8.101 1805.47 176.82 1994.24 2242.84 17 430 458.27 8.018 1987.19 160.75 2163.86 2380.26 18 430 464.11 8.002 1939.24 151.16 2106.52 2310.94 19 430 446.81 8.013 1913.59 152.89 2081.99 2290.19 Week pCO2 aimed for pCO2 obtained [ppm] [ppm] 1 430 477.22 2 430 3 pH 20 430 448.00 8.001 1867.78 145.27 2028.61 2227.57 21 430 466.14 8.003 1952.80 152.62 2121.61 2327.56 22 430 474.71 8.001 1980.46 154.14 2151.09 2358.02 23 430 479.70 8.001 2001.17 155.74 2173.58 2381.94 24 430 465.74 8.012 1992.99 159.10 2168.27 2381.97 25 430 460.06 8.001 1919.23 149.36 2084.57 2287.24 26 430 447.83 8.001 1867.88 145.34 2028.78 2227.84 27 430 471.33 8.002 1969.50 153.53 2139.40 2345.94 28 430 447.92 8.003 1876.06 146.59 2038.21 2238.76 29 430 456.82 8.003 1913.27 149.49 2078.63 2281.76 30 430 460.06 8.001 1919.22 149.36 2084.56 2287.22 31 430 459.08 8.003 1922.72 150.23 2088.89 2292.68 Average control 430.00 444.05 8.022 1933.27 158.06 2106.75 2321.58 Standard deviation 0.00 42.81 0.04 74.78 13.08 77.72 80.96 pCO2 aimed for pCO2 obtained pH HCO3- CO32- DIC TA [ppm] [ppm] [µmol kg-1] [µmol kg-1] [µmol kg-1] [µmol kg-1] 1 430 470.00 8.002 1972.40 153.64 2142.45 2349.03 2 600 653.00 7.871 2121.57 122.31 2267.73 2419.75 3 1000 1007.00 7.720 2199.34 89.61 2323.93 2417.83 4 1000 1022.00 7.715 2207.61 88.96 2332.07 2424.41 5 1000 1002.00 7.739 2129.94 90.58 2252.98 2351.94 6 1000 1000.00 7.728 2225.02 92.36 2352.12 2449.68 7 1000 1200.00 7.652 2240.13 78.01 2359.82 2430.17 8 1000 1100.00 7.692 2249.81 85.84 2373.87 2458.49 9 1000 1034.00 7.712 2216.02 88.60 2340.54 2431.82 10 1000 1012.00 7.728 2248.94 93.23 2377.33 2475.31 11 1000 1002.00 7.725 2214.04 91.26 2340.11 2436.26 Treatment Elevated Week 12 1000 1100.00 7.689 2236.51 84.83 2359.55 2442.96 13 1000 1000.00 7.744 2307.99 99.37 2442.10 2548.03 14 1000 1090.00 7.701 2051.63 79.98 2165.71 2249.31 15 1000 1108.00 7.679 2202.56 81.68 2322.73 2401.96 16 1000 1171.00 7.668 2265.83 81.79 2388.30 2464.57 17 1000 1131.00 7.698 2059.74 79.81 2173.99 2256.87 18 1000 1111.00 7.684 2230.14 83.51 2352.24 2433.52 19 1000 1201.00 7.646 2212.42 76.03 2330.17 2398.09 20 1000 980.00 7.730 2188.66 91.19 2313.89 2411.11 21 1000 1000.00 7.731 2238.60 93.49 2366.82 2465.73 22 1000 1200.00 7.650 2231.19 77.39 2350.26 2419.86 23 1000 1090.00 7.693 2236.22 85.59 2359.67 2444.49 24 1000 1120.00 7.690 2279.72 86.57 2405.19 2489.68 25 1000 1200.00 7.639 2175.83 73.60 2291.12 2356.14 26 1000 1015.00 7.725 2240.35 92.25 2367.86 2464.50 27 1000 1002.00 7.728 2229.56 92.55 2356.91 2454.60 28 1000 800.00 7.808 2139.88 106.78 2274.45 2400.57 29 1000 1100.00 7.690 2238.85 85.01 2362.07 2445.69 30 1000 1100.00 7.696 2052.95 79.14 2166.60 2248.58 31 1000 980.00 7.743 2011.07 86.32 2127.76 2224.90 32 1000 1000.00 7.724 2018.72 82.97 2133.52 2224.24 Average elevated 969.6875 1031.28 7.720 2183.54 89.82 2308.56 2402.81 Standard deviation 5.44 87.11 0.07 84.88 14.73 84.29 79.75 pCO2 aimed for pCO2 obtained pH HCO3 CO3 DIC TA [ppm] [ppm] [µmol kg-1] [µmol kg-1] [µmol kg-1] [µmol kg-1] 450 465.42 1979.44 197.94 157.05 2152.66 Treatment control fluctuating Week 1 8.010 control fluctuating 2 460 487.69 8.008 2063.84 162.93 2243.71 2459.83 control fluctuating 3 480 475.00 8.011 2026.63 161.31 2204.44 2419.85 control fluctuating 4 400 453.25 8.001 1888.26 146.75 2050.76 2250.97 control fluctuating 5 570 598.76 7.891 1936.42 116.83 2074.05 2225.79 control fluctuating 6 450 472.11 8.001 1968.50 153.12 2138.01 2343.99 control fluctuating 7 450 474.50 8.000 1974.23 153.24 2143.95 2349.83 control fluctuating 8 460 469.98 8.005 1978.41 155.37 2150.10 2358.96 control fluctuating 9 430 433.00 8.014 1859.74 149.01 2023.79 2228.76 control fluctuating 10 500 472.85 8.003 1979.89 154.65 2150.97 2358.69 control fluctuating 11 400 430.00 8.049 2003.37 174.12 2192.43 2427.57 control fluctuating 12 630 650.16 7.904 2166.34 134.66 2323.58 2492.84 control fluctuating 13 520 532.70 7.940 1930.48 130.51 2079.50 2252.98 control fluctuating 14 440 452.38 8.029 2013.17 167.13 2196.02 2420.56 control fluctuating 15 450 467.00 8.018 2022.34 163.38 2201.93 2420.57 control fluctuating 16 400 446.56 8.017 1930.12 155.63 2101.26 2312.75 control fluctuating 17 500 538.31 7.937 1937.87 130.14 2086.71 2259.28 control fluctuating 18 500 510.00 7.995 2095.86 160.68 2274.26 2485.63 control fluctuating 19 480 489.44 7.974 1916.67 140.02 2073.70 2262.40 control fluctuating 20 430 440.00 8.026 1940.77 159.70 2115.76 2332.76 control 21 460 474.49 7.980 1886.58 139.94 2043.00 2232.99 fluctuating control fluctuating 22 400 428.99 8.025 1891.97 155.66 2062.53 2275.87 control fluctuating 23 500 478.00 8.026 2109.71 173.71 2300.03 2529.76 control fluctuating 24 560 566.21 7.945 2076.38 142.05 2238.10 2422.59 control fluctuating 25 590 561.74 7.938 2025.97 136.31 2181.80 2359.82 control fluctuating 26 420 420.00 8.051 1964.52 171.42 2150.53 2383.56 control fluctuating 27 630 620.89 7.898 2044.17 125.55 2191.29 2351.86 control fluctuating 28 480 498.34 7.961 1893.15 134.17 2044.63 2225.49 control fluctuating 29 420 435.00 8.041 1988.89 169.64 2173.64 2402.96 control fluctuating 30 460 460.00 7.997 1898.32 146.14 2060.44 2259.26 control fluctuating 31 500 510.00 7.995 2095.91 160.68 2274.31 2485.69 control fluctuating 32 480 497.96 7.961 1892.01 134.11 2043.41 2224.24 average control fluctuating 478.125 490.96 7.989 1980.62 151.77 2085.80 2342.84 standard deviation 62.14 96.85 0.04 176.73 17.13 361.56 97.54 pCO2 aimed for pCO2 obtained pH HCO3- CO32- DIC TA [ppm] [ppm] [µmol kg-1] [µmol kg-1] [µmol kg-1] [µmol kg-1] Treatment Week elevated fluctuating 1 430 456.46 8.069 1972.40 153.64 2142.45 2349.03 elevated fluctuating 2 653 633.80 7.908 2121.57 122.31 2267.73 2419.75 elevated fluctuating 3 870 947.19 7.789 2199.34 89.61 2323.93 2417.83 elevated 4 1200 1266.23 7.668 2207.61 88.96 2332.07 2424.41 fluctuating elevated fluctuating 5 1300 1158.52 7.630 2129.94 90.58 2252.98 2351.94 elevated fluctuating 6 1115 1266.09 7.685 2225.02 92.36 2352.12 2449.68 elevated fluctuating 7 936 968.23 7.768 2240.13 78.01 2359.82 2430.17 elevated fluctuating 8 840 879.13 7.898 2249.81 85.84 2373.87 2458.49 elevated fluctuating 9 1200 1199.95 7.652 2216.02 88.60 2340.54 2431.82 elevated fluctuating 10 1300 1276.32 7.615 2248.94 93.23 2377.33 2475.31 elevated fluctuating 11 718 725.85 7.851 2214.04 91.26 2340.11 2436.26 elevated fluctuating 12 1250 1313.70 7.626 2236.51 84.83 2359.55 2442.96 elevated fluctuating 13 1100 996.15 7.696 2307.99 99.37 2442.10 2548.03 elevated fluctuating 14 938 909.66 7.761 2051.63 79.98 2165.71 2249.31 elevated fluctuating 15 978 978.39 7.732 2202.56 81.68 2322.73 2401.96 elevated fluctuating 16 1400 1298.18 7.600 2265.83 81.79 2388.30 2464.57 elevated fluctuating 17 800 735.41 7.817 2059.74 79.81 2173.99 2256.87 elevated fluctuating 18 1060 1093.57 7.699 2230.14 83.51 2352.24 2433.52 elevated fluctuating 19 1200 1115.58 7.652 2212.42 76.03 2330.17 2398.09 elevated fluctuating 20 1200 1223.44 7.662 2188.66 91.19 2313.89 2411.11 elevated fluctuating 21 1300 1312.05 7.613 2238.60 93.49 2366.82 2465.73 elevated fluctuating 22 741 761.69 7.861 2231.19 77.39 2350.26 2419.86 elevated fluctuating 23 870 878.04 7.793 2236.22 85.59 2359.67 2444.49 163 164 elevated fluctuating 24 1304 1255.67 7.633 2279.72 86.57 2405.19 2489.68 elevated fluctuating 25 702 656.44 7.878 2175.83 73.60 2291.12 2356.14 elevated fluctuating 26 1077 1002.24 7.716 2240.35 92.25 2367.86 2464.50 elevated fluctuating 27 1200 1149.60 7.656 2229.56 92.55 2356.91 2454.60 elevated fluctuating 28 746 777.68 7.843 2139.88 106.78 2274.45 2400.57 elevated fluctuating 29 1500 1450.15 7.571 2238.85 85.01 2362.07 2445.69 elevated fluctuating 30 870 843.87 7.813 2052.95 79.14 2166.60 2248.58 elevated fluctuating 31 1100 1098.26 7.697 2011.07 86.32 2127.76 2224.90 elevated fluctuating 32 560 770.52 7.898 2018.72 82.97 2133.52 2224.24 average elevated fluctuating 985.46 1012.44 7.74 2183.54 89.82 2308.56 2402.81 standard deviation 262.41 244.48 0.12 86.24 14.96 85.64 81.02 165 166 167 168 169 170 171 172 173 174 175 176 177 178 SI Table 3: Summary of comparisons. SH evolved lineages are different from FH evolved lineages (i.e. environmental stability affects evolution of phenotypes). SH evolved lineages are also different from SA evolved lineages, and FH evolved lineages differ from FA evolved lineages. Further, the plastic response is not maintained in either selection environment (SA plastic response differs from SH evolved phenotypes, and FA plastic response differs from FH evolved phenotypes) – we use the difference from the plastic response to estimate how much evolution there has been. For untits or traits see SI Figure 1. * significant (p<0.05), ** highly significant (p<0.01). NS not significant. Compare main manuscript Figure 2. Comparison O2 O2 R:P Size ChloroNile Red Consumption Evolution Ratio phyll a C:N Ratio Trait value SH evolved compared to trait value FH evolved NS * lower NS ** higher ** higher NS ** higher Evolved response SH compared to ambient response SA * higher ** lower * lower NS NS ** higher ** higher Evolved response FH compared to ambient response FA * higher NS NS ** lower * lower ** higher ** lower Evolved response SH compared to plastic response SA ** lower ** lower ** lower NS NS ** higher * lower Evolved response FH compared to plastic response FA NS NS NS ** lower ** lower ** higher ** lower 179 180 181 182 183 184 185 186 187 188 189 190 191 192 193 194 195 196 197 198 199 200 201 202 203 204 205 206 207 208 209 210 211 212 213 214 215 216 217 218 METHODS Additional detailed Methods for measuring phenotypic traits and rationale for chosing fluctuations Rationale for adding a fluctuating environment component: Little is known about how evolving in regimes of environmental fluctuation that select for plasticity affects key traits in primary producers, or whether the phenotypes of populations evolved in stable environments are good predictors of phenotypes evolved in fluctuating environments. For example, populations evolved in fluctuating environments could simply evolve the same mean trait values as those in stable environments, with the plastic populations having an increase in the variance in trait value. In this case, it is the average environment that is important in determining the average phenotype, and experiments carried out in stable environments are probably good predictors of average phenotypes even in fluctuating environments (though there is some indication variation in phenotypes is important in determining community composition, see Menden-Deuer & Rowlett (2014)). In contrast, evolution in a fluctuating environment may select for qualitatively different phenotypes, such that the average environment plays a limited role in determining the average phenotype, and experiments carried out in stable environments may be poor predictors of phenotypic evolution in less stable environments. Experiments carried out in stable environments may be poor predictors of phenotypic evolution in less stable environments when evolution in a fluctuating environment selects for qualitatively different phenotypes, such that the average environment plays a limited role in determining the average phenotype. Oxygen evolution and consumption rates : Samples were acclimated to the respective assay pCO2 for two transfers (a total of ~ 14 generations). This way, we are more likely to rule out measuring instantaneous stress responses that are not specific to changes in pCO2 levels. The protocols for pre-acclimation and acclimation were the same. For oxygen evolution and respiration measurements, 15ml of each sample were gently spun down to increase cell density. We found that spinning down for 15 minutes at 1500g yielded better results than filtrations. Pellets were then suspended in 2ml of medium of the appropriate pCO 2 and oxygen production rates in the light and oxygen consumption rates in the dark measured in a Clark-type oxygen electrode illuminated at 160 - 180 µmol photons m-2 s-1, with stirring, at 18 ± 0.8 ºC. Prior to measurements, oxygen levels in the cultures were reduced to 15% by bubbling with N 2 and N2/ CO2. For oxygen evolution measurements, changes in oxygen concentration were recorded for 6 minutes in the light. For respiration, oxygen concentration was measured for another 6 minutes in the dark. Oxygen consumption by the electrode was determined at the beginning of each measurement. To avoid bias due to circadian rhythms in Ostreococcus, photosynthesis and respiration measurements were always carried out in a 4-8 h window after the beginning of the light period. Furthermore, we estimated bacterial background respiration by using a cell sorter to obtain medium that only contained bacteria, 219 220 221 222 223 224 225 226 227 228 229 230 231 232 233 234 235 236 237 238 but no Ostreococcus. The sample was then treated the same way as a ‘community’ sample containing 239 Carbonate chemistry: 240 241 242 243 244 245 246 247 248 249 250 251 252 253 254 255 256 257 both Ostreococcus and associated bacteria, to estimate bacterial background respiration. Cell size, chlorophyll content, and Nile Red fluorescence. Chlorophyll content, lipid content, and relative cell size were determined using a FACS CANTO (for general protocols see (Petersen et al., 2012; Marie et al., 2005)). The red fluorescence channel was used as a proxy for total chlorophyll content and chlorophyll content per cell was calculated using chlorophyll calibration beads as standards (Bangs Laboratories, Inc.) as well as through comparing to samples of known chlorophyll a content, where chlorophyll had been extracted according to (HolmHansen & Riemann, 1978) The same channel was used to determine polar lipids after a Nile Red stain (la Jara et al.; Guzmán et al., 2009). Samples (200µl) were measured twice, once with and once without the dye, in order to account for the overlap of Nile Red and chlorophyll auto fluorescence. As the cultures were not axenic, we accounted for possible bacterial contribution to the Nile Red signal by setting the gates so that only chlorophyll positive events within an SSC/FSC gate specific to Ostreococcus were considered. Cell size was inferred from the FSC (forward scatter) and comparison to 1µm, 2µm and 5µm calibration beads. Flow cytometry data was also compared to coulter counter measurements for random samples (an average assay including technical replication consists of more than 800 samples, making coulter counter measurements too low-throughput as the main means of determining cell size). The desired CO2 levels in the seawater was established by bubbling the required amount of artificial seawater for at least 24 hours with the appropriate pCO 2, in an incubator that was set to the same pCO2 as the water was bubbled with. Flasks were inoculated quickly and transferred back to the incubator set to the respective selection or assay pCO2. Seawater carbonate chemistry was calculated from pH and alkalinity using the CO2sys software (1998). DIC was measured colourimetrically (Stoll et al., 2001) and Total alkalinity (TA) was inferred from linear Gran-titration plots (Dickson, 1981). DIC and pH samples for all lineages were taken at the beginning and at the end of the selection experiment. Additionally, we measured pH and either alkalinity or DIC before medium was used for a transfer. pH was measured routinely for all samples at the beginning and at the end of a transfer; DIC was measured for a random set of samples at each transfer, and for all samples at the beginning and the end of the selection experiment. Data for each time point in each selection regime was calculated from a minimum of six samples. Total Alkalinity (TA) was measured at the beginning, 100 generations into the experiment and at the end of the selection experiment. All other values were calculated using seacarb within R for 18 ºC and salinity 32. We did not measure TA and DIC for all samples at each transfer, as we had at least 192 samples in ongoing culture at any point during the selection experiment and taking samples at the end of each transfer only would have amounted to at least 20 000 DIC and TA samples. This would have been outwith the scope of this experiment. 258 259 260 261 262 263 264 265 266 267 268 269 270 271 272 273 274 275 276 277 278 279 280 281 282 283 284 285 286 287 288 289 290 291 292 293 294 295 296 297 298 299 300 301 302 Statistics: ANOVA design: ANOVAs were performed on mixed linear model output. Models were built as described in Pinheiro and Bates (2000). We used the lmer and lme4 packages - which are based on similar algorithms but differ in how easily random effects can be specified or p values obtained. Note that in lme degrees of freedom are not calculated the same way as in other statistics software packages. We therefore recalculated degrees of freedom manually. In spite of our balanced and non-hierarchal design, it was necessary to use mixed models, because lineages between treatments were related to each other due to being founded from clones, and to control for any ‘pseudo’ replication arising from batch-culturing the same culture for many hundreds of generations. In lme, when there was only one random effect and one fixed effect the model was specified as random as below: fit2.lme <- anova(lme(variable~fixed effect , random=~+1|random effect)) In lmer, when there were several random effects (for example, in order to include sampling origin), the general code read: lmer(response variable~ fixed effect1*fixed effect2 + (1|random effect1) + (1|random effect2) + (1|random effect 3)) For example, a model testing the Ho that the interaction of assay and selection environment pCO2 do not yield different phenotypes , might read like this (this also tests for differences between biological and technical replicates) m1 <-lmer( traitvalues ~ selected*measured +(1|lineage)+(1|lineage:selected), data = data) m2<lmer(traitvalues~1+(1|Lineage)+(1|Lineage:Selection)+(1|Lineage:Selection:Biorep)+(1|Lineage:S election:Biorep:Techrep), data = data) Correction for multiple comparisons: Even in a simple one-way ANOVA with K group means, there are K(K − 1)/2 pairwise comparisons. Our ANOVAs -with many comparisons - were followed by a Holm-Bonferroni correction for multiple comparisons to protect against inflation of Type 1 errors. Correcting at the level of the ANOVA, this limits the amounts of comparisons carried out in the next step (post-hoc tests). The ‘post hoc’ values reported in the manuscript were obtained from Tukey’s HSD tests, which also control for multiple comparisons. In addition to this we broke down omnibus ANOVAs (for example, an ANOVA assuming as H0 that there was no difference between the means of populations in stable and fluctuating environments) where p-values indicated a significant outcome, into several smaller ANOVAS testing for more specific outcomes on the level of individual traits or lineages from different sampling origins. We are happy to make R code available on request. 303 304 305 306 307 SI References 308 309 Guzmán HM, la Jara Valido de A, Duarte LC, Presmanes KF. (2009). Estimate by means of flow cytometry of variation in composition of fatty acids from Tetraselmis suecica in response to culture Dickson AG. (1981). An exact definition of total alkalinity and a procedure for the estimation of alkalinity and total inorganic carbon from titration data. Deep Sea Research Part A Oceanographic Research Papers 28:609–623. 310 conditions. Aquacult Int 18:189–199. 311 312 Holm-Hansen O, Riemann B. (1978). Chlorophyll a Determination: Improvements in Methodology. Oikos 30:438. 313 314 la Jara de A, Mendoza H, Martel A, Molina C. R. Diaz (2003). Flow cytometric determination of lipid content in a marine dinoflagellate, Crypthecodinium cohnii. 315 316 Marie D, Simon N, Vaulot D. (2005). Phytoplankton Cell Counting by Flow Cytometry. In:Algal Culturing Techniques, Elsevier, pp. 253–267. 317 318 Menden-Deuer S, Rowlett J. (2014). Many ways to stay in the game: individual variability maintains high biodiversity in planktonic microorganisms. J R Soc Interface 11:20140031–20140031. 319 320 Petersen TW, Brent Harrison C, Horner DN, van den Engh G. (2012). Flow cytometric characterization of marine microbes. Methods 57:350–358. 321 322 Schaum CE, Collins S. (2014). Plasticity predicts evolution in a marine alga. Proc Biol Sci 281:20141486–20141486. 323 324 Schaum E, Rost B, Millar AJ, Collins S. (2013). Variation in plastic responses of a globally distributed picoplankton species to ocean acidification. Nature Climate change 3:298–302. 325 326 Stoll MHC, Bakker K, Nobbe GH, Haese RR. (2001). Continuous-Flow Analysis of Dissolved Inorganic Carbon Content in Seawater. Anal Chem 73:4111–4116. 327 328 329 330 (1998). CO2SYS—Program developed for the CO2 system calculations. Carbon Dioxide Inf. Anal. Center. available at http://scholar.google.com/scholar?q=related:Wnqve01Uz4MJ:scholar.google.com/&hl=en&num=30& as_sdt=0,5. 331