File
advertisement

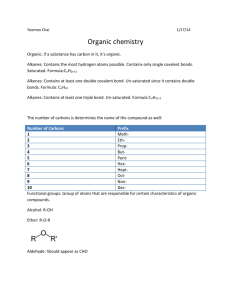
Alkane 1.) Count the longest carbon chain If the carbon chain is: 1 carbon: named "methane" 2 carbons: named "ethane" 3 carbons: named "propane" 4 carbons: named "butane" 5 carbons: named "pentane" 6 carbons: named "hexane" 7 carbons: named "heptane" 8 carbons: named "octane" 9 carbons: named "nonane" 10 carbons: named "decane" Note that organic chemists, being lazy, will often not label hydrogens or carbons. For instance, would be hexane (each of the "points" is assumed to be a carbon) 2.) Number the carbon chain, starting with the end closest to the substituent. Always try to get the lowest number possible. If there are two substituents, name the carbon chain so that it has the lowest possible number.For instance, check out this molecule: Would this compound be numbered from the left or right? The answer is that it would be answered from the right. If the molecule was named from the left, you would have substituents on carbons 3 and 5. Numbering from the right, the subsituents are on carbons 2 and 4. Once you have determined which direction you will name, you will have to write down the molecule. 3.) Name and number the substituent When naming, name the substituent before the parent chain. Name this compound: You would name this 2-"substituent" hexane. You would not name it 5-"substituent" hexane because 2 < 5. These are the common substituents in organic chemistry: (CH3--): methyl (CH3CH2--): ethyl (CH3CH3CH2--): butyl Notice the pattern yet? You take the full name, for instance, hexane, drop the "ane" and add "yl," so a carbon chain with (CH3CH3CH3CH3CH3CH2--) would be called "hexyl" Now, when you have two of the same substituents on a molecule, use these common prefixes: two of the same substituent: di three of the same substituent: tri And so it keeps goin.... Name this compound: 1. The longest carbon chain is 6 carbons. Hence the parent chain is named "hexane" 2. Two subsituents are located on carbons 2 and 3, not 4 and 5. 3. There are two "methyl" substituents 4. Hence, we have the name, 2,3-dimethylhexane If you have two substituents that are not the same, then you must name them both before the parent chain, listed alphabetically. Note that the prefixes used to designate number (i.e. di and tri) do not affect the alphabetization. Name this compound: 1. The longest parents chain is 6 carbons, hence its parent name is "hexane" 2. I spy, with my own eyes, three substituents! There are two methyl and one ethyl group. How should I number them? Well, seeing as to how I can get 2,3,4 instead of 3,4,5, I shall name them from the left. 3. So the substituents are 2,4-dimethyl and 3-ethyl. Note that "e" in ethyl precedes "m" in "methyl. The "di" doesn't affect the alphabetization. 4. Finally, we name the compound as 5. 3-ethyl-2,4-dimethylhexane. Cyclic alkanes Cyclic alkanes and conformations Naming cyclic alkanes First you would count the longest carbon chain. You can see that the "cyclic" ring has 8 carbons, so it's octane. Since it's a cyclic ring, you call it a "cyclo" alkane. Hence, the main part of the molecule would be called cyclooctane. The substituent is called a methyl group, so you get the name methylcyclooctane. Now, the hard part of this is figuring out numberings. You can pick ANY carbon to be "1," so we'll conveniently choose the carbon that the methyl is on as methylcyclooctane. It wouldn't matter if the methyl group was on the top right carbon, because it would still be methylcyclooctane, because you could rotate that molecule to get the one above. Now, let's try two substituents. We again know it's a cyclooctane, but the problem arises in how to number the substituents. We could name this 1-methyl-3-ethylcyclooctane, or 1-ethyl-3-methylcyclooctane. We name it 1-ethyl-3-methylcyclooctane because of the alphabetization rules. Also, priority is given to the longest carbon chain substituent in naming (so we wouldn't name it 3-ethyl-1methylcyclooctane). These are some ways to draw the same compound, cyclohexane. Cyclohexane is a pretty important molecule, and I guarantee you will see these again. Basically the last two are cyclohexane drawn the way it really does look. The 2nd drawing is for "watered down" organic that is taught to AP Chemistry students. If you take a real orgo class (although I've only taken an intro course) you will learn all sorts of cool stuff like that. Alkene Nomenclature of Alkenes Alkenes are hydrocarbons that have double bond(s). In the naming of a hydrocarbon, you use the suffix -ene. For instance, a straight chaine of six carbons would be called hexene. When naming alkenes, you must designate where the double bond occurs. Numbering occurs on the lower numbered carbon. This would be 2-hexene. NOT 3-hexene. 3-hexene would be: This is pretty easy, until you start getting into substituents. Naming substituents in a straight chain (also called an n- chain) is pretty basis. Name this compound: This would be called 2-methyl-3-hexene. In organic, there is a whole "priority" in numbering. If you have a methyl- group and a -ene group, then the -ene group gets priority (meaning that the -ene must the lowest possible). For instance let's look at this molecule: This would be called 4-methylcyclohexene. The -ene group has priority over the methylgroup, so the -ene is assumed to be one. Then, counting in a counterclockwise direction (clockwise would yield 5-methylcyclohexene, and lower numbers are preferred), the methyl group is located on the 4 carbon. Alkenes: Cis/Trans & E/Z Let's take a look at how cis/trans affects alkenes. Are these two molecules the same? Yes they are! Because this is a molecule, you can rotate this. So let's look at it. If you take the first molecule, and rotate it on the x-axis, then rotate it on the y-axis, you get the second molecule. They are the same. They are both 2-propene. Are these two the same? The answer is NO. No matter how many times you rotate the molecule, you will not end up with the same molecules. If you remember from our alkane tutorial, one of these is cis and one of these is trans. Which one is which? The one on the LEFT is trans, because the methyl groups are on the opposite ends. The one on the right is a cis, because the methyl- groups are located on the same side. The molecule on the left is called trans-2-butene, while the right one is called cis-2-butene. A general note: The double bond is rigid and is not able to freely rotate, unlike single bonds. Nomenclature of Alkynes Alkynes are named similarly to alkenes. Much like alkenes, you change the ending of the molecule name to designate a triple bond. Instead of the -ane or the -ene, you use -yne. You number the carbon to the lowest possible carbon number. The above molecule is called 2-pentyne. Alkynes have the lowest priority (other than alkanes, which are THE lowest). This means that when you have both a double bond and triple bond, the double bond gets the priority in numbering. Let's look at this molecule: This molecule is called 1-penten-4-yne. The simplest possible alkyne is the two carbon triple bond, which would be called ethyne. However, in keeping with "historical" names, the name "acetylene" is popular. This isn't anything that you can deduce, it's one of the random names that organic chemists like to use. So, remember that this molecule, is called acetylene (although ethyne is still a good name to use). Alcohol Nomenclature: Alcohols are just like other substituents. There isn't anything real special about them. I'm assuming you've read all the sections before this, so I can breeze through this section. Molecules with alcohols are called -ols. For instance + ol = methanol). is methanol. (methane - ane Among alcohols, there are primary, secondary, and tertiary alcohols. The structure on the left is the primary alcohol. Primary alcohol means that the -OH is attached to a carbon group which has NONE or ONE other carbon attached to it. Secondary alcohols, as demonstrated by the middle structure, has an -OH group attached to a carbon which is attached to two carbons. It is also called a sec-alcohol. Tertiary alcohols, the right structure, is an alcohol with the -OH group attached to a carbon with connections to three other carbons. Tert-alcohols, as they are called, are very bulky. The significance of these comes later on. If you want to know how it stands in terms of functional group priority, hit this link. Name this This is 2,5-heptadiol. No cis/trans becuase it's an alkane. Alkanes have free rotation. Aromatics: Aromatic compounds in organic chemistry are those which smell. They contain conjugated rings of carbons. However, not all conjugated rings are considered aromatic. To determine which compounds are aromatic, follow the Hückel Rule. Notice how the left one only has 4 pi electrons (not enough!) and the right onw has 8 pi electrons (sorry!). So, using Hückel's rule, you can figure out almost all the aromatic compounds. Benzene Benzene is symmetrical, planar, hexagonal strcture that boils at 80 C and melts at 5 C. Benzene is the simplest of the aromatics (n=1 for Hückel's rule). Although you may see benzene written like that, benzene actually exists as two different compounds. Benzene Substituents Two substituents that involve the benzene ring are phenyl- and benzyl- groups. The molecule on the left is the phenyl- group and the one on the right is the benzyl- group. Aromatic groups are sometimes written Ar- (which is short for aryl) while R- represents an alkyl group. So if you saw someting written as Ar-CH3, that would be a methylbenzene (or phenylmethane). Aldehyde Nomenclature: The aldehyde fucntional group is a -CHO which looks like this: The carbonyl carbon (the one connected O and H) is always carbon 1, unless there is a carboxylic acid group on the other end. Hence, the numbers aren't mentioned when mentioning aldehydes. In order to designate a molecule as an aldehyde, you must drop the "ending" (-ane from an alkane) and add "al" (for aldehyde). Name this molecule: That would be butanal. Now, try this one: This would be 3-butenal. Try this one: 2-ethylhex3-ene-4-yne-al. That was a toughie. When the aldehyde isn't the highest priority group, the prefix formyl- is used. Let's try this one: This would be formylpentanoic acid. No need to number, because both the carboxylic and aldehyde functional groups are located at the end, and there are ony two ends :) Ketone Nomenclature: The ketone functional group looks like this: Molecules with the ketone functional group will have the suffix -one. For instance, in this molecule: This would be 2-pentanone. You must number ketones, unlike aldehydes, since the ketone will not be located at the end of a carbon chain. Like always, you try to get the lowest number possible. In terms of functional group priority, ketones take priority over everything but carboxylic groups and aldehydes. So, among all the functional groups that are no located at the end, ketones have highest priority. If an aldehyde or carboxylic functional group is presenta long with the ketone, the ketone becomes an oxo- prefix. So, in the case of this molecule: This would be called 2-oxopropanal. Carboxylic Acid Nomenclature: The carboxylic acid functional group is the highest priority functional group. The -COOH functional group can also be seen here molecularly: When naming a group with a carboxylic functional group, you take the alkane name (for instance, butane), and drop the -ane and add -oic acid. So, butane would be butanoic acid. So name this compound: This would be butanoic acid. Pretty easy. Try this one: This is benzoic acid! See, carboxylic acids are pretty easy. Historical Names The hardest thing about acids is that a lot of them have "historic" names which have pattern. So here are some of them: Structure IUPAC Name Historical Name Methanoic Acid Formic Acid Ethanoic Acid Acetic Acid Propanoic Acid Propionic Acid Butanoic Acid Butyric Acid Amine Nomenclature: Naming an amine is simliar to naming an alcohol. You just take the parent chain (for instance, hexane), drop the -e, and add -amine (hence, you would get hexanamine). Try this one: Yes, that's methanamine. Very easy. Remember tertiary, secondary, and primary structures? Well, in amines, it is important to note which kind of amine you have, because it affects the nomenclature. Now, when a hydrogen on the amine group (-NH2) is replaced with something else, you use N- to designate that it's a substituent of the amine group itself. Let's look at an example: The parent chain is the propane group. The amine is off carbon 2, so we have the name 2butanamine. However, the amine group has had a hydrogen removed and an ethyl added. So, the naming would be N-ethyl-2-butanamine. If both the hydrogens have been removed, you do the N,N- prefix. The only time amines have a prefix (amino-) is when a higher functional group exists on the molecule. Alcohols, ketones, aldehydes, and carboxylic acid groups all have higher priority, so when those are present, the -amine suffix becomes an amino- prefix. Here is an example: The parent chain is a hexane. Now, normally, if the -OH group wasn't there, this would be called hexanamine. However, the -OH has a higher priority, so this molecule becomes 5aminohexan-2-ol. Amine groups on benzenes are called anilines. Other than that, there is normal numbering. Try this molecule: The amine group on the benzene makes this an aniline. The methyl group is ortho to the amine group. So, we get the name o-methylaniline. Amine Reactions: Protonatin of amines to form ammonium salts Alkylation of amines Note that you must have a primary amine and a primary or secondary alkyl bromide for this reaction Alkylation of amines Note that you must have a tertiary amine and a primary or secondary alkyl bromide for this reaction