LAB_The Periodic Table
advertisement

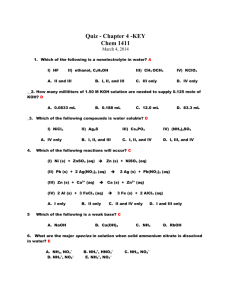
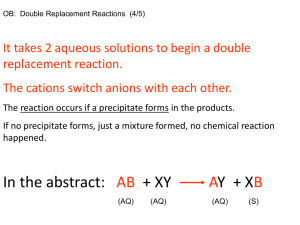
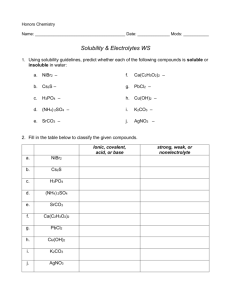
Pg. 1 The Periodic Table Adapted from Chem Fax 4511, Flinn Scientific Inc., 1998 Introduction: The Periodic Table of Elements is one of the most useful tools in chemistry. It organizes more than 100 elements (118 as of 2012) and helps chemists summarize many known facts about them. At-a-glance, it displays the similarities and differences among the elements. In 1869, Dmitri Mendeleev, a Russian chemist organized the known elements at that time (~63 elements) in a table. He organized them in order of increasing atomic weights, moving from left to right in horizontal rows he called periods. By doing so, he found that certain elements repeated similar physical and chemical properties at specific intervals which he then organized into vertical columns called groups/ families. To maintain a consistent pattern or periodicity in the elemental properties, Mendeleev was forced to leave some “gaps” or “holes” in the table. He believed that this was because the holes were created by elements that had yet to be discovered. He was absolutely true! This kit involves four student activities – two paper exercises and two lab procedures that demonstrate the periodic nature of the elements. Activities: #1 – Paper Activity - Fill in the Missing Information #2 – Paper Activity – Element Crossword Puzzle #3 – Lab Activity – The Reactivity of Two Alkaline Earth Metals #4 – Lab Activity – The Solubility of Group II (Alkaline Earth) Metals Name: Activity #1 – Fill in the Missing Information Pg. 2 Directions: Using the Periodic Table as a guide, fill in the missing information on the chart below. SYMBOL NAME ATOMIC # Lithium Sn 16 Hydrogen Fl 35 Argon Sr 55 Neon Se 2 Iron Au 42 Ra Mg 22 Silicon O 92 Chlorine Na 19 Silver P 29 MASS # FAMILY #/Name Name: Per. Tab. Lab Activity #1 Pg. 6 Comparing the Reactivity of Two Alkaline Earth Metals Introduction: Students will investigate the reactivity of two alkaline earth metals by observing both qualitative and quantitative properties. Concepts: Periodicity Reactivity Rates of Reaction Materials – Chemicals and Equipment Chemicals: Magnesium (Mg) Turnings, 2-3 pieces Calcium (Ca), small lump or turnings Hydrochloric acid, 1.0 M, HCl, 2.0 mL Distilled water, H2O *Periodic Table Equipment: Test tube rack and Test tubes (tt), 2 Disposable pipette, 1 Cover (paraffin)/Cap for test tubes Forceps Thermometer Safety: HCl is corrosive to skin and eyes; avoid all contact with body tissues. Wear safety goggles, apron and chemical resistant gloves. Consult teacher for disposal instructions. Equipment: Chemical – resistant gloves/apron Safety goggles Procedure: 1. 2. 3. 4. 5. 6. 7. Using a disposable pipette, add 20 drops (~1mL) of 1.0M HCl to tt#1 and tt#2. Place 2-3 Mg turnings into tt#1 and, at the same time, place 1-2 small pieces of Ca turnings into tt#2. Cover both tt with caps or paraffin wrap. Be sure to use ALL your senses to make observations. Record your observations in the data table below. Continue to watch the reactions for 3 minutes. Remove the covers of the tt and record the temperature of each solution. Record ALL observations in the table. Be sure to compare the rates of reactions in the two tt. Data Table: OBSERVATIONS Visible – Color Changes/Effervescence Auditory – Sounds/if any? Reaction Rate – Slow/Moderate/Rapid Temperature (oC) Mg(s) + HCl(aq) Ca(s) + HCl(aq) Name: Per. Tab. Lab Activity #1 Pg. 7 Post – Lab Questions: 1. Which Group II metal did you find to be more reactive, Mg or Ca? 2. Based upon the position of these 2 elements on the Periodic table, write a general statement about Period vs. Reactivity. 3. Based on your observations in this lab and using the Periodic table, predict which Group II element would be the most reactive – Be, Sr, or Ba? 4. In this lab, you observed the ionization (reactions where ions are formed or change their charge) of two different metals in acid. When ionization occurs, the solid metal loses electrons to form the aqueous metal cation (positively charged ions) creating a halide salt. For example, the half reactions are written as: Mgo(s) → Mg+2(aq) + 2e- Cao(s) → Ca+2(aq) + 2e- The energy needed to cause this ionization to occur is called the ionization energy of the element. The higher the ionization energy, the harder it is for the atom to lose electrons. Based on this information, which metal do you predict would have a higher ionization energy, Mg or Ca and why? 5. How would tearing the Mg into even smaller pieces affect the Rate of Reaction (Speed up/Slow down/No effect) and Why? 6. List at least 2 other factors (besides particle size) that may affect the Rate of Reaction? 7. Write the complete balanced word and chemical equation for the reaction of magnesium and hydrochloric acid. Word Equation: Chemical Equation: 8. Write the complete balanced word and chemical equation for the reaction of calcium and hydrochloric acid. Word Equation: Chemical Equation: Name: Per. Tab. Lab Activity #2 Pg. 8 Analyzing the Solubility of Group II Metals Introduction: Students will investigate the trends in solubility of alkaline earth metal (Group II) cations. Concepts: Periodicity Solubility Materials – Chemicals and Equipment Chemicals: Magnesium Nitrate, 0.2M, Mg(NO3)2, 1-2 drops/well Calcium Nitrate, 0.1M, Ca(NO3)2, 1-2 drops/well Strontium Nitrate, 0.1M, Sr(NO3)2, 1-2 drops/well Barium Nitrate, 0.1M, Ba(NO3)2, 1-2 drops/well Ammonium oxalate, 0.2M, (NH4)2C2O4, 1-2 drops/well Potassium chromate, 0.2M, K2Cr2O4, 1-2 drops/well Ammonium sulfate, 1.0M, (NH4)2SO4, 1-2 drops/well Ammonium hydroxide, 3.0M, (NH4)2OH, 1-2 drops/well Ammonium carbonate, 2.0M, (NH4)2CO3, 1-2 drops/well Equipment: 24-well plate Disposable pipette, 9 *Periodic Table Safety: (NH4)2OH is TOXIC by ingestion and inhalation. It is very irritating to the eyes and respiratory tract. K2Cr2O4 is HIGHLY TOXIC and is corrosive to skin, eyes and respiratory tract. Strontium and Barium compounds are TOXIC by ingestion. Oxalates are TOXIC by inhalation and ingestion and are irritating to body tissues. Students MUST wear safety goggles and aprons, chemical – resistant gloves and apron Equipment: Chemical – resistant gloves/apron Safety goggles Procedure: 1. Place a 24-well plate on the lab bench on top of a black sheet of paper. 2. Place 1-2 drops of Mg(NO3)2 into 5 wells in a vertical column (e.g., Column A – Data Table). Do the same with the Ca(NO3)2, Sr(NO3)2, and Ba(NO3)2 in subsequent vertical columns. See Data Table. 3. Now place 1-2 drops of Ammonium Oxalate, (NH4)2C2O4, to the 4 wells containing the 4 different nitrates across the same horizontal row (Row 1). OBSERVE the reactions in each well. RECORD your observations in the BOXES in the Data Table. 1. Now place 1-2 drops of K2Cr2O4 to the 4 wells containing the 4 different nitrates across the same horizontal row (Row 2). OBSERVE the reactions in each well. RECORD your observations in the BOXES in the Data Table. 2. Now place 1-2 drops of (NH4)2SO4 to the 4 wells containing the 4 different nitrates across the same horizontal row (Row 3). OBSERVE the reactions in each well. RECORD your observations in the BOXES in the Data Table. 3. Now place 1-2 drops of (NH4)2OH to the 4 wells containing the 4 different nitrates across the same horizontal row (Row 4). OBSERVE the reactions in each well. RECORD your observations in the BOXES in the Data Table. 4. Now place 1-2 drops of (NH4)2CO3 to the 4 wells containing the 4 different nitrates across the same horizontal row (Row 5). OBSERVE the reactions in each well. RECORD your observations in the BOXES in the Data Table. Name: Per. Tab. Lab Activity #2 Pg. 9 Data Table Record your observations in the BOXES in the table below – they each represent the corresponding well on the 24-well plate. List any COLORS that are formed. If any solid forms (precipitates), use the abbreviation PPT. If NO REACTION at all occurs, use NR. Note: Be sure to make careful observations since the reactions with aqueous ammonium hydroxide may be difficult to see. Chemical Name Chemical Formula Row # Ammonium Oxalate, (NH4)2C2O4 Potassium Chromate, K2Cr2O4 Ammonium Sulfate, (NH4)2SO4 Ammonium Hydroxide, (NH4)2OH Ammonium Carbonate, (NH4)2CO3 Magnesium Nitrate, Mg(NO3)2 Calcium Nitrate, Ca(NO3)2 Strontium Nitrate, Sr(NO3)2 Barium Nitrate, Ba(NO3)2 Col A Col B Col C Col D 1 2 3 4 5 Post Lab – Questions 1. A color change or the formation of a precipitate is an indication that a chemical reaction has taken place. Which Group II metal (Mg, Ca, Sr, or Ba) showed a reaction with the MOST compounds? 2. Which Group II metal was the least reactive? 3. List the 4 Group II metals in order of increasing reactivity, from the least reactive to the most reactive. (Least reactive) (Most reactive) 4. Compare the order to the position of the elements on the Periodic table and write a general statement regarding reactivity and position in a group or family. Name: Per. Tab. Lab Activity #2 Pg. 10 Post Lab Extension Questions: For each of the 20 chemical reactions that took place in Lab Activity #2: a) Write a balanced equation for each reaction that occurred. b) Write the net ionic equation for each reaction that formed a precipitate. (Use the solubility table to determine if a precipitate formed). Examples: Full Equation 1Ba(NO3)2(aq) + 1(NH4)2C2O4(aq) → 1BaC2O4(s) + 2NH4NO3(aq) Net Ionic Equation Ba+2(aq) Full Equation _Mg(NO3)2(aq) + _(NH4)2C2O4(aq) → _Mg(NO3)2(aq) + _ K2Cr2O4(aq) → _Mg(NO3)2(aq) + _(NH4)2SO4(aq) → _Mg(NO3)2(aq) + _(NH4)2OH(aq) → _Mg(NO3)2(aq) + _(NH4)2CO3(aq) → _Ca(NO3)2(aq) + _(NH4)2C2O4(aq) → _Ca(NO3)2(aq) + _ K2Cr2O4(aq) → _Ca(NO3)2(aq) + _(NH4)2SO4(aq) → _Ca(NO3)2(aq) + _(NH4)2OH(aq) → _Ca(NO3)2(aq) + _(NH4)2CO3(aq) → _Sr(NO3)2(aq) + _(NH4)2C2O4(aq) → _Sr(NO3)2(aq) + _ K2Cr2O4(aq) → _Sr(NO3)2(aq) + _(NH4)2SO4(aq) → _Sr(NO3)2(aq) + _(NH4)2OH(aq) → _Sr(NO3)2(aq) + _(NH4)2CO3(aq) → _Ba(NO3)2(aq) + _(NH4)2C2O4(aq) → _Ba(NO3)2(aq) + _ K2Cr2O4(aq) → _Ba(NO3)2(aq) + _(NH4)2SO4(aq) → _Ba(NO3)2(aq) + _(NH4)2OH(aq) → _Ba(NO3)2(aq) + _(NH4)2CO3(aq) → + C2O4-2(aq) BaC2O4(s) Net Ionic Equation