Set2ans
advertisement

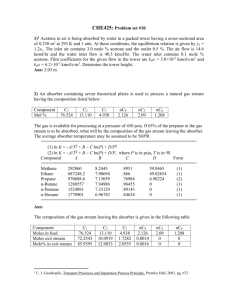
CHE425: Problem set #2 1. A 3-ft depth of stagnant water at 25oC lies on top of a 0.10-in. thickness of NaCl. At time t < 0, the water is pure. At time t = 0, the salt begins to dissolve into the water. If the concentration of salt in the water at the solid-liquid interface is maintained at saturation (0.00544 mol NaCl/cm3) and the diffusivity of NaCl is 1.2×10-5 cm2/s, independent of concentration, estimate, by assuming the water to act as a semi-infinite medium, the time and of salt in the water when: (a) 10% of the salt has dissolved; (b) 50% of the salt has dissolved; and (c) 90% of the salt has dissolved. Density of NaCl = 2.165 g/cm3. Molecular weight of NaCl = 58.44 g/mol. Part (a) 10% transferred (b) 50% transferred (c) 90% transferred Moles transferred 0.000941 0.004705 0.008469 Time, s 1,960 48,900 159,000 Time, h 0.544 13.6 44.0 2. A slab of dry wood of 4-inch thickness and sealed edges is exposed to air of 40% relative humidity. Assuming that the two unsealed surfaces of the wood immediately jump to an equilibrium moisture content of 10 lb H2O per 100 lb of dry wood, determine the time for the moisture content at the center of the slab to reach 1% of the equilibrium value. Assume a diffusivity of water of 8.3×10-6 cm2/s. 0.01 x 1.82 t, s 234,000 Time, h 65 3) Dry air at 1 atm flows at 2 m/s across the surface of a 2-inch-long surface that is covered with a thin film of water. If the air and water are at 25oC and the diffusivity of water in air is 0.25 cm2/s, estimate the water flux in kmol/sm2 for the evaporation of water at the middle of the surface, assuming laminar boundary-layer flow. Air at 25oC, = 0.018 cP = 1.8 x 10-5 kg/m-s. nH2O A (0.016)[0.00128 0] 2.05 105 kmol/s-m2 4. Air at 1 atm and 100oC flows across a thin, flat plate of subliming naphthalene that is 1 m long. The Reynolds number at the trailing edge of the plate is at the upper limit for a laminar boundary layer. Estimate: (a) the average rate of sublimation in kmol/sm2; and (b) the local rate of sublimation 0.5 m from the leading edge. Vapor pressure of naphthalene = 10 torr; viscosity of air = 0.0215 cP; molar density of air = 0.0327 kmol/m3; and diffusivity of naphthalene in air = 0.94×10-5 m2/s. Solution c h nA,B / A 0.0059 4.3 102 0 2.54 104 kmol / s - m2 nA,B / A kcx cA i cA o 0.00417 4.3 102 0 1.79 104 kmol/s-m 2 5. Water at 25oC flows turbulently at 5 ft/s through a straight, cylindrical tube cast from benzoic acid, of 2-in inside diameter. If the tube is 10 ft long, and fully developed, turbulent is assumed, estimate the average concentration of acid in the water leaving the tube. The properties are: solubility of benzoic acid in water = 0.0034 g/cm3; viscosity of water = 0.89 cP = 0.0089 g/cms; and diffusivity of benzoic acid in water at infinite dilution = 9.18×10-6 cm2/s. (0.00367)(4,870) 3 cAout =0.0034 1 exp = 0.000020 g/cm (152)(20.2 5) Therefore, the concentration of benzoic acid in the exiting water is way below the solubility value. 6.2 Air at 1 atm flows at a Reynolds number of 50,000 normal to a long, circular, 1-in diameter made of naphthalene. Calculate the average sublimation flux in kmol/sm2. Vapor pressure of naphthalene = 10 torr; viscosity of air = 0.0215 cP; molar density of air = 0.0327 kmol/m3; and diffusivity of naphthalene in air = 0.94×10-5 m2/s. N A kc cAs cA (0.080)(4.3 104 0) 3.44 105 kmol/s-m2 7. Carbon dioxide is stripped from water by air in a wetted-wall tube. At a location where pressure is 10 atm and temperature 25oC, the flux of CO2 is 1.62 lbmol/hft2. The partial pressure of CO2 is 8.2 atm at the interface and 0.1 atm in the bulk gas. The diffusivity of CO2 in air at these conditions is 1.6×10-2 cm2/s. Assuming turbulent flow, calculate by film theory the mass-transfer coefficient kc for the gas phase and the film thickness. kc (0.00022)(0.475) 0.315 cm/s (0.000409) 0.82 0.01 = DAB / kc = 1.6 x 10-2/0.315 = 0.051 cm 8. Water is used to remove CO2 from air by absorption in a column packed with Pall rings. At a region of the column where the partial pressure of CO2 at the interface is 150 psia and the concentration in the bulk liquid is negligible, the absorption rate is 0.017 lbmol/hft2. The CO2 diffusivity in water is 2.0×10-5 cm2/s. Henry’s law for CO2 is p = Hx, where H = 9,000 psia. Calculate by film theory the mass-transfer coefficient kc in cm/s for the gas phase and the film thickness in cm. kc 2.31 106 0.0025 cm/s (0.0556) 0.0167 0 = DAB /kc = 2 x 10-5/0.0025 = 0.0080 cm Ref: J. D. Seader and E. J. Henley, Separation Process Principles , Wiley, 2011