LentzFinal
advertisement

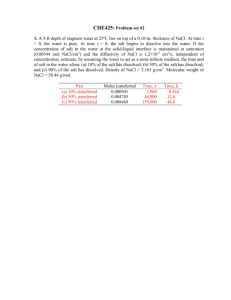
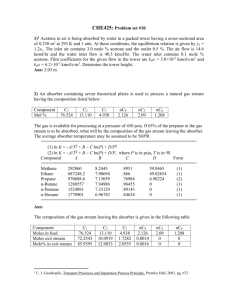
1. Octane (C8H18) is burned with theoretical amount of air at a pressure of 500 kPa. Determine (a) the air fuel ratio on a mole basis, (b) the air fuel ratio on a mass basis, (c) If the products are cooled at a constant pressure of 100 kPa, at what temperature (in deg-C) will dew start to form? (d) Suppose the products are cooled way below the dew point temperature so that all the water from the products are removed (neglect any vapor which may still be present at very low amount). What would be the volume fraction of CO2 in the dry products? (e) If you have a smog test report of your gasoline burning car at hand, compare your result with an actual measurement (write about it in your written submission). Since Octane is burning with theoretical air, it becomes necessary to first determine the chemical balance equation: 𝐶8 𝐻18 + 𝑎(𝑂2 + 3.76𝑁2 ) → 𝑏𝐶𝑂2 + 𝑐𝐻2 𝑂 + 𝑎 ∗ 3.76𝑁2 (1) To determine our coefficients, we can balance the numerical values: Balancing the Carbon: 8=𝑏 (2) 18 = 2𝑐 => 𝑐 = 9 (3) Balancing the Hydrogen: And finally balancing the Oxygen (this should always be done last): 2𝑎 = 2 ∗ 𝑏 + 𝑐 => 𝑎 = 12.5 (4) Since this is a stoichiometric reaction, we can directly write our final chemical equation by combining equations (1)-(4): 𝐶8 𝐻18 + 12.5(𝑂2 + 3.76𝑁2 ) → 8𝐶𝑂2 + 9𝐻2 𝑂 + 47𝑁2 (5) (a) To determine the AF ratio on a molar basis, we can directly use equation (5) to determine the AF ratio: kmol 𝐴𝐹𝑚𝑜𝑙𝑎𝑟 = kmol Air = Fuel 12.5(1+3.76) 1 kmol = 59.5 kmol Air Fuel (6) (b) To determine the AF ratio on a mass basis, we will use the molar mass of both the air and the fuel to convert (6) to a mass basis: kmolAir ∗MAir 𝐴𝐹𝑀𝑎𝑠𝑠 = kmol 29 kg kg = 59.5 ∗ 114.23 kg Air = 15.105 kg Air Fuel ∗MFuel Fuel (7) Fuel (c) The dew point temperature is defined as: 𝑇𝐷𝑃 = 𝑇𝑆𝑎𝑡@𝑃𝑉 (8) Or in words, the dew point temperature is the saturation temperature at the partial pressure of the vapor. In order to determine the partial pressure, we must determine the molar ratio of the H2O: 𝑦𝐻2 𝑂 = 𝑛 𝑛𝐻2 𝑂 𝑃 𝑚𝑖𝑥𝑡𝑢𝑟𝑒 9 = 𝑃 𝑉 = 8+9+47 = 0.1406 (9) 𝑡𝑜𝑡 This implies that the partial pressure of the vapor is: 𝑃𝑉 = 𝑦𝐻2 𝑂 ∗ 𝑃𝑡𝑜𝑡 = 0.1406 ∗ 100 = 14.06 (10) From TEST with the found pressure and a quality of 1: 𝑇𝐷𝑃 = 𝑇𝑆𝑎𝑡@𝑃𝑉 = 52.6C (11) (d) If all the water would be removed, we would now have the following species present in our products: 8𝐶𝑂2 + 47𝑁2 (12) The volume fraction can be determined from the mole fraction: 𝑦𝐶𝑂2 = 𝑛 𝑛𝐶𝑂2 𝑚𝑖𝑥𝑡𝑢𝑟𝑒 = 𝑉𝐶𝑂2 𝑉𝑡𝑜𝑡 8 = 8+47 = 0.145 (13) (e) Compare the results of (d) with a smog report. From my 1996 Honda accord that has 200k miles, the following was outputted at 15 mph: Test 15MPH %CO2 14.8 %O2 0.0 HC (PPM)/% 17/.0017 %CO 0.48 NO PPM/% 46/.0046 From the above data, it appears that the CO2 production is almost exactly that found in (13). This is interesting as there are clearly many other species in the product. And the combustion of gasoline is very difficult to model. However, to analyze the CO2 present, clearly octane may be used to determine the percentage of CO2 present if water is removed. 2. One kmol of ethane (C2H6) is burned with an unknown amount of air. If the combustion is assumed complete and there are 2 kmol of free O2 in the products, determine (a) the percent theoretical air used, (b) the excess air used (in percent), (c) the equivalence ratio. As before, we must first determine the chemical balance for a theoretical mixture: 𝐶2 𝐻6 + 𝑎(𝑂2 + 3.76𝑁2 ) → 𝑏𝐶𝑂2 + 𝑐𝐻2 𝑂 + 𝑎 ∗ 3.76𝑁2 (1) Balancing the Carbon: 2=𝑏 (2) 6 = 2𝑐 => 𝑐 = 3 (3) 2𝑎 = 2𝑏 + 𝑐 = 4 + 3 = 7 => 𝑎 = 3.5 (4) Balancing the Hydrogen: Balancing the Oxygen: Combining (1)-(4) into one equation: 𝐶8 𝐻6 + 3.5(𝑂2 + 3.76𝑁2 ) → 2𝐶𝑂2 + 3𝐻2 𝑂 + 13.16𝑁2 (5) (a) To determine the amount of theoretical air, we must assume that the reaction is complete: 𝐶2 𝐻6 + 𝜆 ∗ 3.5(𝑂2 + 3.76𝑁2 ) → 2𝐶𝑂2 + 3𝐻2 𝑂 + 13.16𝑁2 + 2𝑂2 (6) Where 𝛼 is the theoretical air percentage. This can be determined from balancing the Oxygen in the equation: 𝜆 ∗ 3.5 ∗ 2 = 2 ∗ 2 + 3 + 2 ∗ 2 = 11 (7) Which implies that the theoretical air: 𝜆 = 1.57 = 157% (8) (b) From the above equation, we can determine the percentage of excess air: 𝜆 = 1 + 𝑥 = 1.57 (9) Where x is the percent excess air. Solving; 𝑥 = 57% (10) (c) To determine the equivalence ratio, we can use the following expression: 1 1 𝜙 = 𝛼 = 1.57 = .6369 (11) 3. Carbon is burned with dry air. The volumetric analysis of the products produces 10.06 percent CO2, 0.42 percent CO, and 10.69 percent O2 (and the rest N2). Determine the percentage of theoretical air used. Prior to determining the percent theoretical air, we must first determine the stoichiometric relationship: 𝐶 + 𝑎(𝑂2 + 3.76𝑁2 ) → 𝑏𝐶𝑂2 + 𝑎 ∗ 3.76𝑁2 (1) There is no water present in the reaction as there is no hydrogen reacting in the products. Balancing the carbon: 1=𝑏 (2) 2𝑎 = 2𝑏 => 𝑎 = 1 (3) Balancing the Oxygen: For theoretical air we have the following reaction: 𝐶 + (𝑂2 + 3.76𝑁2 ) → 𝐶𝑂2 + 3.76𝑁2 (4) Now a chemical balance must be performed two ways. The first is to balance the carbon as it was given: 𝑎𝐶 + 𝑏(𝑂2 + 3.76𝑁2 ) → 10.06𝐶𝑂2 + 0.42𝐶𝑂 + 10.69𝑂2 + 78.83𝑁2 (5) Balancing the carbon: 𝑎 = 10.06 + 0.42 = 10.48 (6) 2𝑏 = 2 ∗ 10.06 + 0.42 + 2 ∗ 10.69 (7) 𝑏 = 20.96 (8) 10.48𝐶 + 20.96(𝑂2 + 3.76𝑁2 ) → 10.06𝐶𝑂2 + 0.42𝐶𝑂 + 10.69𝑂2 + 78.83𝑁2 (9) Balancing the oxygen: The above yields the following: Dividing through by 10.48 to get in terms of a per fuel reaction: 𝐶 + 2(𝑂2 + 3.76𝑁2 ) → 0.9593𝐶𝑂2 + .04𝐶𝑂 + 1.019𝑂2 + 7.517𝑁2 (10) This yields a percentage of theoretical air of: 𝜆 = 200% (11) Alternatively, the above can be repeated with the same results balancing the following equation: 𝐶 + 𝑎(𝑂2 + 3.76𝑁2 ) → 𝑏(10.06𝐶𝑂2 + 0.42𝐶𝑂 + 10.69𝑂2 + 78.83𝑁2 ) (12) 𝑎=2 (13) 𝑏 = .0954 (14) Solving: This also implies that the theoretical air is 200%. 4. Evaluate (a) Δh–o in MJ/kmol (of CO), (b) Δg–o in MJ/kmol, and (c) lnK at 298 K for the reaction CO + 1/2O2 ↔ CO2 at 1 atm. 1 𝐶𝑂 + 2 𝐶𝑂 ↔ 𝐶𝑂2 (1) (a) To calculate the enthalpy of combustion for the above reaction, we will use the following: 𝑂 𝑂 Δh𝑓𝑂 = h𝑓,𝑃 − h𝑓,𝑅 (2) From Table G from TEST: 𝑘𝐽 MJ Δh𝑓𝑂 = −393,520 − (−110,530) = −282,990 𝑘𝑚𝑜𝑙 = −282.9 kmol (3) (b) Likewise, for the change in Gibbs-function, or the energy of the reaction: Δg 𝑂𝑓 = g 𝑂𝑓,𝑃 − g 𝑂𝑓,𝑅 (4) Again, from Table G, as we are at 298K: 𝑘𝐽 MJ Δg 𝑂𝑓 = −394,360 − (−137,150) = −257,210 𝑘𝑚𝑜𝑙 = −257.210 kmol (5) (c) To determine the ln(K), we first use the definition of ln(K): ln(𝐾) = Δg𝑂 𝑓 𝑅̅ 𝑇 257210 = − 8.314∗298 [ kJ kmol kJ ∗k kmol∗k ln(𝐾) = −103.82 = unitless] (6) (7) Since this is a common reaction, we can use table G from TEST to verify this calculation: ln(𝐾) = −103.762 (8) To one decimal place, these answers are identical. 5. Determine the adiabatic flame temperature (in K) for octane (C8H18) burning with 20% excess air using the IG model. The inlet conditions are 100 kPa, and 298 K. Prior to determining the adiabatic flame temperature, we must determine the chemical reaction. First we determine the theoretical reaction: 𝐶8 𝐻18 + 𝑎(𝑂2 + 3.76𝑁2 ) → 𝑏𝐶𝑂2 + 𝑐𝐻2 𝑂 + 𝑎 ∗ 3.76𝑁2 (1) To determine our coefficients, we can balance the numerical values: Balancing the Carbon: 8=𝑏 (2) 18 = 2𝑐 => 𝑐 = 9 (3) Balancing the Hydrogen: And finally balancing the Oxygen (this should always be done last: 2𝑎 = 2 ∗ 𝑏 + 𝑐 => 𝑎 = 12.5 (4) This implies that the theoretical reaction is: 𝐶8 𝐻18 + 12.5(𝑂2 + 3.76𝑁2 ) → 8𝐶𝑂2 + 9𝐻2 𝑂 + 47𝑁2 (5) Now we can determine the reaction with 20% excess air: 𝐶8 𝐻18 + 12.5 ∗ 1.20(𝑂2 + 3.76𝑁2 ) → 8𝐶𝑂2 + 9𝐻2 𝑂 + 47𝑁2 + 𝑐𝑂2 (6) Balancing the oxygen: 2 ∗ 12.5 ∗ 1.20 = 2 ∗ 8 + 9 + 2𝑐 (7) 𝑐 = 2.5 (8) 𝐶8 𝐻18 + 12.5 ∗ 1.20(𝑂2 + 3.76𝑁2 ) → 8𝐶𝑂2 + 9𝐻2 𝑂 + 47𝑁2 + 2.5𝑂2 (9) The complete reaction: For the adiabatic flame temperature, we must solve the following equation: ∑ ℎ𝑟 = ∑ ℎ𝑝 (10) Using TEST and the IG combustion daemon, this becomes very easy to calculate. After running the calculation with the given initial conditions: 𝑇𝑎𝑓 = 2138K (11) 6. Consider the dissociation of 1 kmol of A2 through the elementary step A2 ↔ 2A. If x stands for the degree of dissociation (fraction of 2 by volume that is dissociated), the overall reaction can be represented by A2 -> (1 - x) A2 + 2x A. At 500 K and 100 kPa, the equilibrium constant is calculated as 1. (a) Determine x . (b) What would be the value of x if the pressure is reduced to 0.01 kPa. (c) Plot how x varies as the total pressure is changed from 0.01 kPa to 1 MPa (make the x axis logarithmic. Obviously, this graph cannot be sumbitted through classTA. Make it part of your final.docx file that you are going to email to homeworksubmission@gmail.com). There are two reactions present. First the partial reaction: 𝐴2 ↔ 2𝐴 (1) 𝐴2 → (1 − 𝑥)𝐴2 + 2𝑥𝐴 (2) The overall reaction becomes: Where x is wholly dependent on the pressure and the equilibrium constant K. The two can be related together via the following equation for the intermediate reaction: 𝜐𝑝 𝐾= 𝜋𝑃 𝑃𝑃 𝜐 𝜋𝑅 𝑃𝑅 𝑅 1 ∗ (𝑃 )∑ 𝜐𝑝 −∑ 𝜐𝑅 0 (3) Where the pi’s are the product of the partial pressures of the products and reactions. (a) Because of the squatted nature of the numerator, the pressure can be factored out and the ratio of pressures can be described. The mole fractions then replace the partial pressures of the reaction: 𝜋𝑃 (𝑃𝑣𝑝 )2 𝐾=𝜋 1 ∗ (𝑃 )2−1 (5) ∗ 12−1 = 1 (6) 1 𝑅 (𝑃𝑣𝑅 ) 0 (5) Simplifies to the following: 𝐾= 2𝑥 2 ) 1+𝑥 1−𝑥 ( ) 1+𝑥 ( The mole fractions come from the overall reaction, however the coefficients come from the intermediate reaction. Solving for x, this becomes: 5𝑥 2 − 1 = 0 (7) 𝑥 = 0.447 = 44.7% (8) For a pressure of 100kPa, x become: Of 57.7% dissociation at this pressure. (b) we must repeat the above reaction for a pressure of .1kPa. K is only dependent on temperature, so it can be assumed to remain constant: 𝐾= 2𝑥 2 ) 1+𝑥 1−𝑥 ( ) 1+𝑥 ( 0.1 2−1 ∗ 100 =1 (9) Solving for x yields: 1.004𝑥 2 − 1 = 0 (10) 𝑥 = 0.998 = 99.8% (11) This implies that: Or at this pressure, 100% of the species disassociates. (c) Plot how x changes with pressure from .1kPa to 1MPa. To solve this, we need to first solve the base equation: 1 4𝑥 2 = 𝛼 (1 − 𝑥)(1 = 𝑥) (12) Where 𝛼 is the ratio of mixture pressure to ambient pressure. This simplifies to: 1 𝑥 = √1+4𝛼 When this relationship is plotted, we achieve the following graph: (13) Dissociation Vs. Pressure 0.8 0.7 Dissociation (x) 0.6 0.5 0.4 0.3 0.2 0.1 0 0 200 400 600 Pressure Ratio) Figure 1. Dissociation vs. Pressure Plotting the above with a log-scale x-axis: 800 1000 1200 Dissociation Vs. Pressure 0.8 0.7 Dissociation (x) 0.6 0.5 0.4 0.3 0.2 0.1 0 10 100 Pressure Ratio) Figure 2. Log scale graph of Figure 1. Note that it is much more linear. This graph implies that the dissociation increases as the pressure goes down. This is nature removing any gradient that is present in the reaction from the above equations. 1000 Dissociation (x) Dissociation Vs. Pressure 0.1 0.1 1 10 Pressure Ratio) Figure 2. Dissociation data on a log-log scale. 100 1000