Good Student Shot Lab
advertisement

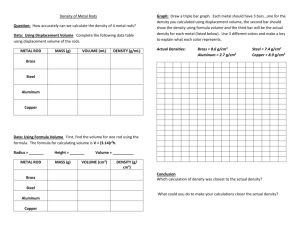
Anonymos (name removed by instructor) Mrs. Trine H. Chemistry P.8 My comments/changes are in bold red type This was a well prepared lab report…good job! Title: Density of Metals Purpose: Determine the density of a given metal. Materials: -Two varieties of metal shot -Water -Electric Balances -Calculator -Graduated Cylinders -Clean-up Materials Pre-Lab Questions: 1. The volume is 40.30ml. 2. The volume is 73 cm3 because1 ml equals 1cm3 in volume. 3. 73.0mL-40.3 mL =32.7 mL volume of the metal : 32.7 cm3 4. Mass- 13.875 g/ volume- 5.143 cm3 =2.6978 g/cm3 2.698 g/cm3 The identity of the metal is aluminum. Great job showing work on prelab questions where possible…be sure to include units at all times though and your volume measurement should have been to the tenths place each time. cm3, should have the 3 as a superscript, not in line with regular text. Procedure: 1. Weigh the Mass of the metal shot on an electric balance. 2. After weighing the massing of the metal shot, pour some metal into the graduated cylinder metal shot, and weigh the mass of the metal and metal shot. 3. Subtract the mass of the metal shot from the total mass of the metal and metal shot together, to get the mass of just the metal. 4. Pour approximately 20 ml of water into the graduated cylinder and record its exact volume. 5. In the graduated cylinder with 20 ml of water, Put in the metal from the metal shot into the graduated cylinder . 6. Find the difference of the level of the water before, and after the metal, to determine the volume of the metal. 7. After finding the mass and volume of the metal, determine the density using the density formula. ( mass divided by over volume) 8. Repeat procedure for additional types of metal shot if time allows. Data Table for Copper Metal: Mass of Metal (g) Volume of Metal (mL) Density of metal (g/cm3) 1 2 3 5.89g 16.56 g 51.2 g 0.5 ml 2.0 ml 5.5 ml 11.8g/cm3 8.1 g/cm3 9.3 g/cm3 4 71.55 g 8.0 ml 8.9 g/cm3 Trial # Qualities Qualitative data: brownish, grayish coloring, tiny spheres, dull Formula: Average density: 9.525 g/cm3 Data Table for Zinc: (see formatting changes made to copper’s table) Trial # Mass of metal 1 5g 2 10g 3 20g 4 50g Qualitive data: circular, shiny, pancake shape Volume of metal 10.1 ml 10.2 ml 10.3 ml 10.7 ml Density of metal 0.5 g/cm3 1 g/cm3 1.9 g/cm3 4.7 g/cm3 Formula used in data table: Average density: 2.025 g/cm3 Post lab: Percent error calculations: (|actual value – guess estimated value| / actual value) x 100 Copper: | 8.96 g/cm3-9.525 g/cm3|= 0.565 g/cm3/8.96 g/cm3=0.063058036 x100= percent error: 6.31% Zinc: |6.57 g/cm3-2.025 g/cm3|=4.545 g/cm3/6.057 g/cm3=0.691780822x100= percent error: 69.2% GRAPH SHOULD BE HERE AND LOOK LIKE THE EXAMPLE WE WORKED ON IN CLASS FOR Au & Pt Show work for the post-lab Post Lab Questions: questions too… 1. The volume is 225.344 units?. 2. The volume is 804 cm3. 3. No, the metal is not copper because the density is what…?. Conclusion: The objective of this lab was to determine the density of a metal with the given materials. Using the given materials and the proper formulas, the objective was met properly. The procedure of the lab wasn’t extremely complex. Density can be found by dividing a sample’s mass by its volume with a formula that has individual measures, and in order to narrow it down to the density, each measure was needed to solve for density. The individual measurements made in this experiment were the volume and the mass of the metals. In order to measure get the volume, a the graduated cylinder filled with a small quantity of water was needed, and to measure get the mass a the scale balance was used needed. Once all the different parts of the formula were found, all that was left was to actually solve the equation of mass over volume determines density. The overall results for this lab were that the density of the copper, metal number 1 was 9.525g/cm3 and metal number #3’s density was 2.025g/cm3 which was Z zinc. While performing this lab timinge was there an encountered a problem. Due to the and shortage of time, there was only time for the collection of data for copper. The data for zinc was collected by Aiden Piper and his lab partner who were kind enough to share the information. The key concepts of this lab were that in order to find the density of a metal, you must first make sure that you have the volume and the mass of the metal must be determined. Those two measurements are extremely needed in order to get the density of any object. This experiment could have been improved by using better estimates, and also having a better more time frame. Over all In summary, the percent error from the density of copper was 6.31% which is reasonable for the quality of equipment available. isn’t that much of a difference which meant that the lab succeeded mostly. On the other hand, However, the results for zinc were indicated a 69.2% error which meanst that the this part of the lab wasn’t didn’t succeed as successful much. In conclusion, in order to define the density of a metal was successfully determined by measuring you must first gather the mass and the volume of several samples of the metal. Very good for a first attempt at formal conclusion writing. Things missing include a discussion of the graph and why multiple trials were analyzed and sources of error in the data. Every experiment has error, whether you make mistakes or not. Always discuss any possible errors thoroughly, including how they would affect final results. Also be careful of not letting any “you’s” slip into formal science writing. Good job listing only the most important results and connecting it to in class learning.