Minerals and Rock Cycle study guide _Repaired
advertisement

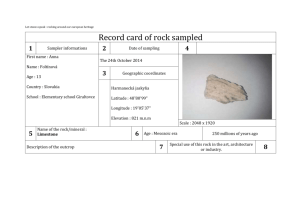
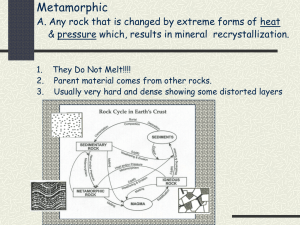
Minerals and Rock Cycle Study Guide Mineral – naturally formed, inorganic solid that has a definite crystalline structure. How to determine if an object is a mineral: 1. Is it a non-living material? Inorganic – non living things 2. Is it a solid? A mineral cannot be a gas or liquid 3. Does it have a crystalline structure? Repeating inner structure reflected in the shape of the crystal. Has a definite (same) chemical composition 4. Is it formed in nature? Materials made by humans are not minerals Understanding Crystals Crystals are solids whose atoms, ions or molecules are arranged in a definite pattern. They have geometric forms. The arrangement depends on the kind of atoms in the crystal. Each mineral has a definite crystal structure that helps classification : Isometric (cubic) Non-isometric (non cubic) Two main groups of minerals 1. Silicate minerals Minerals that contain a combination of silicon, oxygen, and one or more metals. They make up more than 90% of Earths crust Most common are: Quartz Mica Feldspar 2. Non silicate minerals Minerals that do not contain compounds of silicon and oxygen. Some contain carbon, fluorine and sulfur Classes of non silicate minerals: Native elements – composed of only one element (Examples: Copper, Gold, Silver) Carbonates – contain combinations of carbon and oxygen (Example: Calcite) Halides – form when a halogen (fluoride, chlorine, iodine or bromine) combine with a metal like sodium, calcium or potassium. (Example: Fluorite, Halite) Oxides – form when an element like aluminum or iron combine chemically with oxygen. (Examples: Hematite, Corundum, Magentite) Sulfates – contain the ion sulfate, SO4. (Examples: Gypsum, Baryte) Sulfides – contain one or more elements combined with sulfur. (Examples: Galena) Identifying Minerals Properties that help identify a mineral. Property Color luster streak Cleavage Fracture Hardness Density Special properties Description absorption of light (wavelengths), it is the least useful way of identification. how light is reflected from the surface. Two types: metallic or nonmetallic (pearly, resinous, greasy, silky) color of the powder residue on a streak plate after the mineral is scraped. A mineral with hardness of 7 and up does not leave residue tendency of a mineral to break along smooth, flat surfaces. tendency of some minerals to break unevenly along curved or irregular surfaces ability to resist scratching, scientists use the Mohs hardness scale where talc has a rating of 1 and a diamond has a rating of 10. ratio of object’s mass to its volume Specific gravity: ratio of objects density to the density of water. Properties particular to only a few types of minerals. May need special equipment. Fluorescence (glow under UV light – calcite, fluorite) Chemical reaction (fizz or bubble when exposed to acid – calcite) Optical properties ( Taste ( Halite has a salty taste) Magnetism ( magnetite is a natural magnet that attracts iron) Radioactivity (minerals that contain uranium or radium) Striations ( visible parallel lines on the cleavage planes) Smell (sulfur smells like rotten eggs) Rock – naturally occurring solid material made up of one or more minerals. They are always forming and changing. Importance or Value of rocks – important natural resource used since the early years. Early years – hammers, arrowheads, knives Today – buildings, monuments, roads Three main classes: Igneous, Metamorphic, Sedimentary Rock cycle The continual process by which new rock forms from old rock material. Series of processes in which a rock forms, changes from one type to another, is destroyed, and forms again by geological processes. Processes that shape earth Weathering – breaking down rock by physical or chemical processes. o Water, wind, ice and heat break down rock into fragments, which are sediments that form sedimentary rock. Erosion – transport of weathered rock to a new location. Sediment is removed from its source; water, wind, ice or gravity can erode and move the sediment and cause it to collect. Deposition – the process in which material is laid down. When sediment moved by erosion is dropped and comes to rest. Sediment is deposited in bodies of water and other low lying areas. Heat and Pressure – usually work together to alter the rocks under the Earth’s surface. o Sedimentary rock can form when sediment gets squeezed by the weight of overlying layers of sediment. o Enough temperature and pressure can change it into metamorphic rock. Some cases the rock gets hot enough it melts becoming magma. When the magma cools it becomes igneous rock. Weathering and erosion Sedimentary rock Igneous rock E r o si o n cooling magma Heat and pressure metamorphic rock Melting Rock Classification We know we can divide rocks in the three main classes, but we can still divide them further based on the way or place they form. Scientists use two important criteria: Composition – the chemical makeup of a rock; describes the minerals or other minerals in the rock. (example: limestone is made of 95% calcite and 5% aragonite) Texture – the quality of a rock that is based on sizes, shapes, and positions of the rock’s grains. Examples: fine-grained, medium-grained and coarse –grained) Types of rocks 1. Igneous rock – forms when magma (liquid rock) cools and hardens. The type of igneous rock depends on the composition of the magma and where it cools. Examples: Basalt, Granite a. Intrusive igneous rock – rock formed from the cooling and solidification of magma beneath the Earth’s surface. b. Extrusive igneous rock – rock that forms from magma that erupts onto Earth’s surface. Commomn around volcanoes. 2. Sedimentary rock – forms at or near the Earth’s surface when sediment deposits in layers (strata) over layers and get compacted. Their structure include ripple marks, mud cracks and raindrop impressions. It forms without heat and pressure that forms the other two types of rock. a. Clastic – rock or mineral fragments cemented together. (Example: sandstone) b. Chemical – forms from solutions of dissolved minerals and water. (Example: Halite) c. Organic – forms from the remains of plants and animals (fossils). (Example: Coral, Limestone) 3. Metamorphic – rocks in which the structure, texture, or composition of the rock have changed. All three types of rock can be changed by heat, pressure, or combination of both. The minerals in the rock change into new minerals, more stable under new pressure and temperature. a. Foliated - has mineral crystals aligned in bands. (Example: slate, gneiss) b. Non-foliated – have unaligned mineral crystals. (Examples: marble, quartzite)