Page - Springer Static Content Server
advertisement

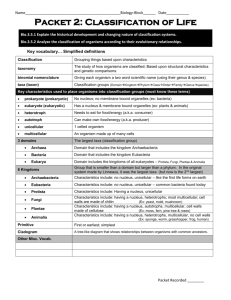
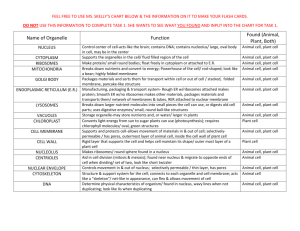
PAGE 1 Supplemental data Methods An autopsy was performed at Hamamatsu Rosai Hospital. In addition to routine neuropathological examination, a diastase digestion test was carried out with periodic acid-Schiff (PAS) staining using paraffin-embedded sections from the brain and liver. Immunohistochemistry for phosphorylated tau (AT8, mouse monoclonal, 1:1,000, Innogenetics, Ghent, Belgium), LC-3, a marker for macroautophagy (rabbit polyclonal, 1:1,000, abcam, Cambridge, UK), ubiquitin (rabbit polyclonal, 1:200, Dako, Glostrup, Denmark), cathepsin D, a lysosomal enzyme (rabbit polyclonal, 1:1,000, Dr. Uchiyama), phosphorylated -synuclein (mouse monoclonal, 1:5,000, #64, WAKO, Osaka, Japan), -amyloid (rabbit polyclonal, 1:100, IBL, Fujioka, Japan), glial fibrillary acidic protein (GFAP, mouse monoclonal, 1:800, DAKO) and CD68 (PG-M1, mouse monoclonal, 1:100, DAKO) was performed with Vectastain ABC Kit (Vector Laboratories, Burlingame, CA). For -amyloid immunohistochemistry, the sections were pretreated with 99% formic acid (WAKO) for 5 min at room temperature. For ultrastructural studies, paraffin-embedded sections were deparaffinized, rehydrated and fixed with 1% osmium, then dehydrated and embedded in Epon (TAAB Epon812, Berkshirek, UK). Ultrathin sections were examined using a Tecnai Spirit transmission electron microscope (FEI, Hillsboro, OR). Acknowledgements We thank Dr. Yasuni Nakamura and Dr. Yasunori Sato for their diagnosis of the liver pathology, Dr. H. Brent Clark for useful discussion, and Mr. Robert Debold for English editing. This work was supported in part by JSPS KAKENHI Grant Number 23590244 (A.F.) and 24300131 (K.W.) from the Ministry of Health, Education, Culture, Sports, Science and Technology, Japan; a Grant-in-Aid for Scientific Research (K.W.) from the Japan Society for the Promotion of Science; Grants-in-Aid for Scientific Research from the Program for Promotion of Fundamental Studies in Health Sciences of the National Institute of Biomedical Innovation, Japan (K.W.). PAGE 2 Supplemental Table 1 Lipofuscin-like granules Pale granular neurons Frontal neocortex + + Hippocampus ++ - Caudate-putamen ++ - Globus pallidus ++ - Claustrum +++ - Thalamus ++ - Nucleus basalis of Meynert ++ +++ Oculomotor nucleus ++ +++ Red nucleus + - Substantia nigra + - Locus ceruleus + - Pontine nucleus ++ - Dorsal vagal nucleus + ++ Hypoglossal nucleus + ++ Inferior olivary nucleus +++ - Cerebellar Purkinje cells - - -, none; +, mild (<5%); ++, moderate (5-30%); +++, severe (>30%). PAGE 3 Supplemental Figure 1 Immunohistochemistry for LC3, a marker for macroautophagy (a-d) and ubiquitin (e-h) in the basal nucleus of Meynert (a, b, e, f) and cardiac muscles (c, d, g, h) from the control case (a, c, e, g) and Danon disease (b, d, f, h). Pale granular neurons in the basal nucleus are not immunoreactive for LC3 (arrows in b). Ubiquitin immunoreactivity is slightly increased in pale granular neurons (arrows in f). In contrast to the brain, immunoreactivity for both LC3 (arrowhead in d) and ubiquitin (arrowheads in h) is increased in the vacuolated cardiomyocytes. Bars 250 m (a-h). PAGE 4 Supplemental Figure 2 Hypertrophic and dilated cardiomyopathy. a Foci of whitish patchy fibrosis (arrows) in the hypertrophic myocardium (615 gram). b Extensive fibrosis in the myocardium. HE. c, d Immunoreactivity for LC3 (arrowheads in c) and cathepsin D (arrowheads in d) is increased in the marginal zone of fibrosis. Bars 2 cm (a) and 200 µm (b-d). PAGE 5 Supplemental Figure 3 Liver cirrhosis with nodular lesions. a Two demarcated nodular lesions with 2.3 and 1 cm in diameter (arrows and inset) in the left lobe of the enlarged liver (1620 gram). b, d Nodular lesions consisting of regenerative nodules with non-neoplasitc clear and dark hepatocytes, and thin septal and portal region, suggesting focal nodular hyperplasia-like region. c, e Background liver tissue showing micronodular liver cirrhosis. b, c Azan, d, e HE. f-h Electron microscopy showing electron-dense granular structures in the perikaryal cytoplasm (arrows and insets). Bars 2 cm (a), 1 cm (inset of a), 2 mm (b, c), 50 µm (d, e) 3 µm (f-h) and 500 nm (insets of f-h).