Comparing Enthalpy Changes Between Different Alcohols to
advertisement

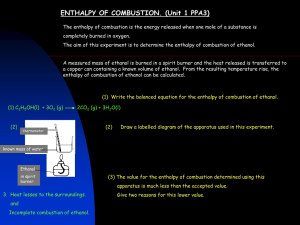
Jean Zhang Best Fuel Lab: Comparing Enthalpy Changes of Different Alcohols to Determine Fuel Efficiency Introduction: The purpose of this experiment is to determine which of two types of alcohols, methanol (CH3OH) or ethanol (CH3CH2OH), is the more efficient fuel for human use. The higher the enthalpy change of combustion of an alcohol (measured in part by the temperature change of water caused by the alcohol), the better it is as a fuel. Balanced equations for the combustion of methanol and ethanol: Methanol combustion: CH3OH(l)+ O2 (g) --> CO2 (g)+ 2 H2O(l) +heat energy Ethanol combustion: CH3CH2OH(l)+ 3 O2 (g) --> 2 CO2 (g) + 3 H2O(l)+heat energy Independent variable- two types of alcohol, methanol and ethanol Dependent variable- temperature change of water caused by heat released in the combustion of the alcohol, the enthalpy change, the amount of alcohol burned Controlled/constant variables- the amount of water (which will be heated), the distance from the tip of the alcohol burner (the wick) to the beaker of water, time alcohol burned Materials: Safety Materials: Goggles Apron Lab Materials: Provided alcohol burner containing methanol (over 150g) Provided alcohol burner containing ethanol (over 150g) 100ml of distilled water A 250ml beaker A 250ml graduated cylinder A stirring rod A pipette A scientific thermometer An electronic balance A ring stand Wire Gauze Matches or a lighter Stopwatch Procedures: Jean Zhang 1. Measure 100ml of distilled water into a 250ml graduated cylinder and pour into a 250ml beaker. 2. Take the provided alcohol burner containing at least 150g of methanol. Measure and record the total mass (in grams) of the methanol and the alcohol burner with a balance. 3. Set up the ring stand and place the wire gauze on top of the ring clamp. 4. Place the beaker of 100ml of water on top of the wire gauze Adjust the ring clamp so that when the alcohol burner is placed under the clamp, the wick of the burner is 10cm from the wire gauze. 5. Measure and record the temperature of the water with a thermometer. 6. Use a match or lighter to light the wick of the alcohol burner containing the methanol. Stir the water in the beaker continuously with a stirring rod as it is being heated. 7. Use a stopwatch to time the burning of the methanol. Put out the fire after 3 minutes of burning. 8. Measure and record the final total mass of the alcohol burner and the methanol with a balance. Measure and record the final temperature of the distilled water with a thermometer. 5. Repeat steps 1-8 for ethanol. Do three trials for each alcohol. 6. Organize all data. 7. Calculate the change in temperature of the water. Calculate the mass burned of each alcohol by subtracting the final total mass from the initial total mass for each. Take the average of the data for the two trials for both methanol and ethanol. 8. Calculate the enthalpy change of the combustion of each alcohol using the given formula: Δ H= (mass)(specific heat capacity)(change in temperature)/ number of moles. Mass refers to the mass of the water, the heat capacity is the specific heat capacity of water (4.187 J/g*K), and the change in temperature is that of the water. Use the chemical equation for each alcohol to determine the number of moles, which can be found by dividing the mass burned of each alcohol by its molar mass. 9. Compare the results. Compare the amount of alcohol burned. Whichever alcohol produced the higher enthalpy change is the one that is the better fuel. Note that a negative sign for an enthalpy change value indicates an exothermic reaction. Data Collection: Methanol Trial 1 Methanol Trial 2 Methanol Trial 3 Ethanol Trial 1 Ethanol Trial 2 Ethanol Trial 3 Initial Temp of Water (C) Final Temp of Water (C) 18.30.1 19.60.1 18.70.1 18.90.1 18.50.1 17.70.1 37.40.1 40.10.1 41.70.1 34.20.1 34.60.1 33.20.1 Initial Mass of Burner + Alcohol (g) 173.3290.001 170.0470.001 166.7930.001 187.1150.001 185.7710.001 184.4490.001 Final Mass of Burner + Alcohol (g) 170.0470.001 166.7930.001 163.5590.001 185.7710.001 184.4490.001 183.1430.001 Jean Zhang Data Processing: Methanol Trial 1: ΔT=37.4C-18.3C=19.1K Δ H= Methanol Trial 2: ΔT=40.1C-19.6C=20.5 K Δ H= Methanol Trial 3: ΔT=41.7C-18.7C=23.0 K Jean Zhang Δ H= Ethanol Trial 1: ΔT=34.2C-18.9C=15.3 K Δ H= Ethanol Trial 2: ΔT=34.6C-18.5C=16.1 K Δ H= Ethanol Trial 3: ΔT=33.2C-17.7C=15.5 K Δ H= Jean Zhang Average of Δ H: Average Δ H of Methanol= Average Δ H of Ethanol= Percentage Error: % error for Δ H of Methanol = (|Experimental Result - Accepted Value| / Accepted Value) x 100=(|86.1-725|)/(725)=88.1% % error for Δ H of Ethanol = (|Experimental Result - Accepted Value| / Accepted Value) x 100=(|228-1364|)/(1364)=83.3% Conclusion: The experiment achieves the aim of determining which alcohol, methanol or ethanol, would be a better fuel by testing and comparing their respective enthalpy changes. Though the experiment was not valid, it was fairly reliable (more so than valid), suggesting that the same or similar human error was involved in producing the percentage errors. Thus the experiment does successfully determine whether methanol or ethanol is the better fuel, but does not correctly determine their enthalpy changes. The relationship between the independent variable, the type of alcohol, and the dependent variable, the amount of alcohol burned, shows that in the same duration of time, 3 minutes in all three trials, less ethanol than methanol is used up when it is burned. Experimenting with methanol, the water was raised by 19.1C, 20.5C and 23.0C in methanol trials 1, 2 and 3 respectively, and for ethanol the water was raised by 15.3C, 16.1C and 15.5C in ethanol trials 1, 2 and 3 respectively. This shows roughly a 30% higher temperature change in the water heated in the methanol trials compared to that of the ethanol trials. The average mass of alcohol burned for each methanol trial was, however, almost 150% the amount burned in the ethanol trials. Analyzing the above, it can be inferred that even under the same circumstances (same amount of energy available: 3 minute burning fire in each trial), for a certain mass (and moles, in this case) of alcohol, ethanol will emit more energy than methanol. Thus its enthalpy change will be higher (since a negative sign only indicates an exothermic reaction). Even though the accuracy was low, the results of each trial are consistent in showing the definite Jean Zhang relationship between the type of alcohol and the mass burned; ethanol burns slower than methanol in each case. Evaluation: Comparing the expected and experimental results for the enthalpy change of methanol, the percentage error for our actual result was 88.1%, far lower than the expected value, suggesting that heat was lost in the experiment. The enthalpy change of ethanol had quite a similar percentage error, at 83.3%, this value was again considerably lower than the expected value; the heat energy released by the ethanol probably was not fully transferred to the water. A possible reason for this difference is because of the heat loss due to using a beaker to hold the heated water. With such a wide opening, heat is easily released from the top, greatly lowering the water’s potential to rise in temperature. Another reason heat might have been lost is because of the distance of the flames from the beaker. To ensure that the distance of the ring clamp was kept constant, the flames were not moved closer to the beaker, causing the beaker to not fully be heated. In order to produce better results, the change from using a beaker to hold the water to an aluminum can (as used in a previous experiment) should be made. This decreases the surface area from which heat can be lost. Also, the ring clamp should be adjusted so that the distance from the tip of the flame to the bottom of the container is the same in each trial, and closer to the bottom. Such measures should be taken to minimize the heat energy loss that would decrease the validity.