***** **** 9
advertisement
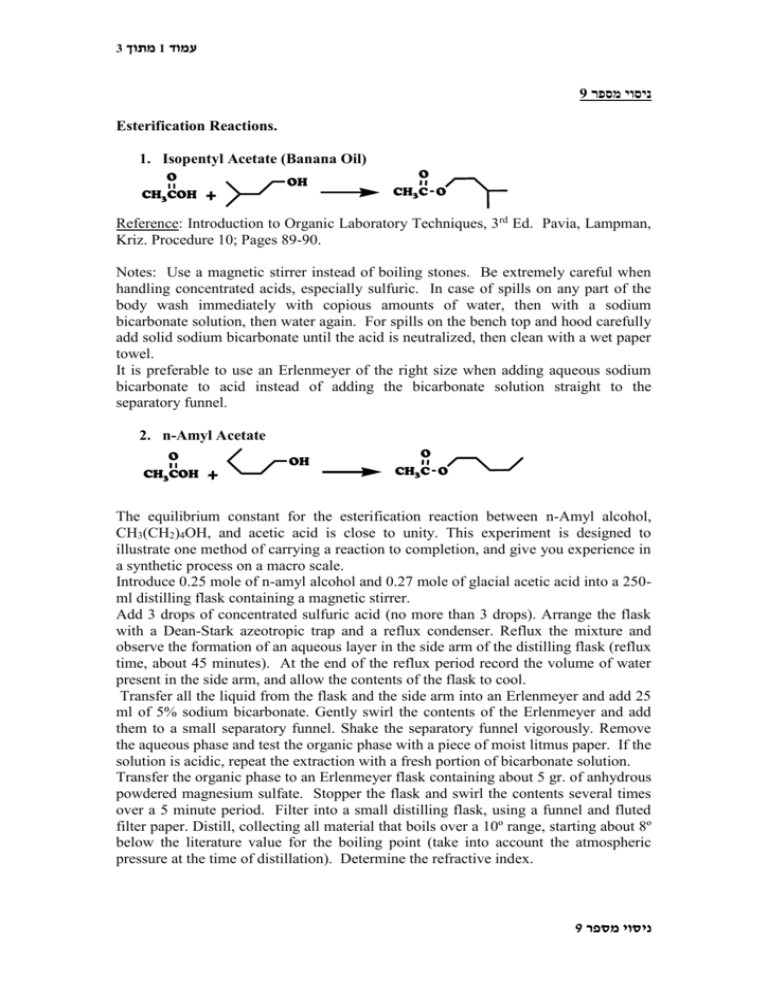
3 מתוך1 עמוד 9 ניסוי מספר Esterification Reactions. 1. Isopentyl Acetate (Banana Oil) O CH3COH + OH O CH3C O Reference: Introduction to Organic Laboratory Techniques, 3rd Ed. Pavia, Lampman, Kriz. Procedure 10; Pages 89-90. Notes: Use a magnetic stirrer instead of boiling stones. Be extremely careful when handling concentrated acids, especially sulfuric. In case of spills on any part of the body wash immediately with copious amounts of water, then with a sodium bicarbonate solution, then water again. For spills on the bench top and hood carefully add solid sodium bicarbonate until the acid is neutralized, then clean with a wet paper towel. It is preferable to use an Erlenmeyer of the right size when adding aqueous sodium bicarbonate to acid instead of adding the bicarbonate solution straight to the separatory funnel. 2. n-Amyl Acetate O CH3COH + OH O CH3C O The equilibrium constant for the esterification reaction between n-Amyl alcohol, CH3(CH2)4OH, and acetic acid is close to unity. This experiment is designed to illustrate one method of carrying a reaction to completion, and give you experience in a synthetic process on a macro scale. Introduce 0.25 mole of n-amyl alcohol and 0.27 mole of glacial acetic acid into a 250ml distilling flask containing a magnetic stirrer. Add 3 drops of concentrated sulfuric acid (no more than 3 drops). Arrange the flask with a Dean-Stark azeotropic trap and a reflux condenser. Reflux the mixture and observe the formation of an aqueous layer in the side arm of the distilling flask (reflux time, about 45 minutes). At the end of the reflux period record the volume of water present in the side arm, and allow the contents of the flask to cool. Transfer all the liquid from the flask and the side arm into an Erlenmeyer and add 25 ml of 5% sodium bicarbonate. Gently swirl the contents of the Erlenmeyer and add them to a small separatory funnel. Shake the separatory funnel vigorously. Remove the aqueous phase and test the organic phase with a piece of moist litmus paper. If the solution is acidic, repeat the extraction with a fresh portion of bicarbonate solution. Transfer the organic phase to an Erlenmeyer flask containing about 5 gr. of anhydrous powdered magnesium sulfate. Stopper the flask and swirl the contents several times over a 5 minute period. Filter into a small distilling flask, using a funnel and fluted filter paper. Distill, collecting all material that boils over a 10º range, starting about 8º below the literature value for the boiling point (take into account the atmospheric pressure at the time of distillation). Determine the refractive index. 9 ניסוי מספר 3 מתוך2 עמוד 3. Methyl Benzoate CO2H + CO2Me CH3OH Place 10 gr of benzoic acid and 25 ml of methanol in a 100 ml round-bottomed flask, and pour 3 ml of concd. sulfuric acid slowly and carefully down the walls of the flask and then swirl to mix the components. Add a magnetic stirrer, attach a reflux condenser, and reflux the mixture gently for 1 hour. Cool the solution and decant it into a separatory funnel containing 50 ml of water. Add 35 ml of CH2Cl2 using part to rinse the original reaction flask. Shake, and drain off the water (which removes most of the sulfuric acid and methanol). Wash with a second portion of water (25 ml) drain, and then wash with 25 ml of 5% sodium bicarbonate to remove unreacted benzoic acid. Shake, with frequent release of pressure, until no further reaction is apparent; then drain off and acidify the water layer. Any benzoic acid that precipitates is collected, dried and the amount recovered used to calculate the overall yield. Repeat the washing with sodium bicarbonate until no precipitate forms on acidification of the aqueous layer. Wash with water, then dry over sodium sulfate or magnesium sulfate and filter. Remove the solvent by distillation on a rotary evaporator or through a condenser from a distilling flask heated on the steam bath, and collect the solvent in a receiver chilled in ice. Decant the methyl benzoate into a 50 ml distilling flask and distill under reduced pressure using the equipment supplied in the laboratory for this purpose. Note both the distillation temperature and pressure and compare to literature values by using a pressure temperature nomograph. Remember to use a “cow” distilling adapter to be able to change receiving flasks while under low pressure. Remember also to pre-weigh your receiving flasks to calculate the yield. 4. n-Butyl Proprionate O O OH + OH O In a 250 ml round bottomed flask are placed 7.4 gr of propionic acid, 22.2 gr (28.8 ml) of n-butyl alcohol, 0.25 gr of p-toluene-sulphonic acid and 60 ml of toluene. The flask is provided with an azeotropic Dean-Stark trap and a reflux condenser. When the mixture is heated on a heating bath, droplets of water begin to appear in the trap. The refluxing is continued until the liquid in the trap consists of two clear layers (about two hours). About four ml of water are formed. The solution remaining in the flask is washed with water, sodium bicarbonate solution, again with water, dried over MgSO4 and the toluene is removed by distillation followed by the distillation of butyl propionate. Isolate your compound and note the distillation temperature. Don’t forget to add a calcium chloride trap to your distillation apparatus to prevent the contamination of butyl propionate with moisture. Record the index of refraction of your ester. 9 ניסוי מספר 3 מתוך3 עמוד 5. Methyl Salicylate CO2H OH + CH3OH CO2Me OH Reference: Introduction to Organic Laboratory Techniques, 3rd Ed. Pavia, Lampman, Kriz. Procedure 11; Pages 91-94. Vacuum distillation. 9 ניסוי מספר











