F-13 IMPACT OF ANTIBIOTICS ON INTERNATIONAL NORMALIZED
advertisement

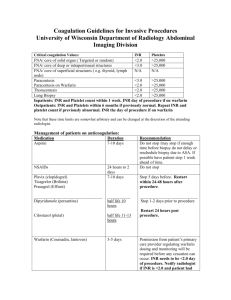
F-13 IMPACT OF ANTIBIOTICS ON INTERNATIONAL NORMALIZED RATIO IN ELDERLY, STABLE WARFARIN PATIENTS Patolia D*, Martin K, James M, Cohen H Kingsbrook Jewish Medical Center, Brooklyn, NY 11203, Arnold & Marie Schwartz College of Pharmacy and Health Sciences, Long Island University, Brooklyn, NY 11201 Objective: Various classes of drugs, including antibiotics, have been shown to interact with warfarin to cause an increase in the international normalized ratio (INR). The clinical relevance of warfarin-antibiotic interactions in older adults is not well studied. The aim of this study was to determine the effect of select antibiotics on the INR in elderly patients on stable warfarin therapy. The primary objective was to calculate the percent change of INR due to the warfarin-antibiotic interaction and the secondary objectives were to determine the occurrence of supratherapeutic INRs > 3.5 and bleeding events. Methods: This was a retrospective, single center, chart review study in acute and long-term care patients conducted at Kingsbrook Jewish Medical Center (KJMC). Data were collected from January 2010 through November 2012. Included were adults 65 years and older on uninterrupted warfarin therapy with a stable, non-supratherapeutic pre-antibiotic INR. The patient must have been started on one or more of the following antimicrobial agents: levofloxacin, ciprofloxacin, piperacillin-tazobactam, trimethoprim-sulfamethoxazole, metronidazole, and fluconazole and a post-antibiotic INR was required to be obtained within a week of initiation of antibiotics. Exclusion criteria were recent start of warfarin, change in warfarin dose after the pre-antibiotic recorded INR, and interrupted warfarin therapy in the prior 5 days before antibiotic initiation. Patient demographics and laboratory information including hospital admission diagnosis, comorbidities, coagulation markers, and concurrent medications were obtained from computerized medical records and the pharmacy application (Siemens®), and bleeding events were reviewed through the KJMC Adverse Drug Event database. Results: The percent change of INR due to the warfarin-antibiotic interaction as well as occurrence of supratherapeutic INRs > 3.5 and bleeding events will be presented. Conclusion: It is anticipated that this project will clarify the magnitude of INR increase from the warfarin-antibiotic interaction. This will provide practitioners additional data to determine whether pre-emptive warfarin dose adjustments are necessary when initiating a specific antibiotic.