5 Homemade fertilisers - teacher notes
advertisement

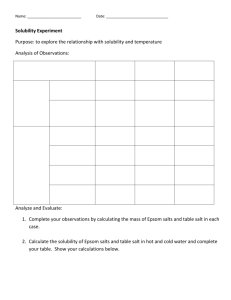
Homemade fertilisers Technical & Teaching Notes Related sheets Plants, matter and energy; Plant reactions; Plant nutrients; Making and testing nutrients; Soil culture; Water culture; Floating culture Introduction and context Many commercial fertilisers are available in gardening stores and garden centres for use at home. However, there are also many websites that give advice and suggestions for making homemade fertilisers. Students read the introduction to an article from Gardening Know How about making fertilisers at home. Homemade Plant Food: Organic Plant Food Recipes To Make At Home “Plant fertilizer purchased from the local garden nursery often has chemicals that not only may harm your plants, but are not environmentally friendly. They don’t sound particularly edible either. In addition, they can be a bit pricey. For this reason, many gardeners are making plant food themselves using organic plant food recipes.” The article goes on to describe some ‘organic plant food recipes’, including ‘Homemade plant food’ and ‘Epsom salts plant fertiliser’. Students follow the instructions to make their own ‘homemade fertilisers’. Possible barriers to learning The session allows a number of common misconceptions and other hindrances to learning to be addressed, including: Fertilisers are plant food Plants get their food from the soil ‘Salts’ are the same as salt (sodium chloride) Links to National curriculum for science in England at key stage 3 In biology pupils should be taught about nutrition and digestion, which includes: plants making carbohydrates in their leaves by photosynthesis and gaining mineral nutrients and water from the soil via their roots In chemistry pupils should be taught about the particulate nature of matter and about atoms, elements and compounds. This includes: differences between atoms, elements and compounds Safety Notes Household ammonia is typically 5-10% w/v, which is about 3-6 mol dm-3. At these concentrations it is an irritant, affecting the eyes and skin. Students should wear eye protection. If any of the solution is spilt or splashes on to the skin, it should be washed off under a running tap. Other substances used are low hazards. Science & Plants for Schools: www.saps.org.uk Homemade Fertilisers: p. 1 Equipment and materials These are only needed for Part 3: Testing ‘Epsom salts plant fertiliser’ if students carry out the investigation. If they do, students will need a teaspoon a plastic bucket (or other container that can hold 4 dm 3 of water), though if the quantities are scaled down a smaller container can be used wooden stick or plastic rod to stir the mixture baking powder (a mixture of sodium hydrogen carbonate and flour, but just sodium hydrogen carbonate will be fine) Epsom salts (magnesium sulfate) saltpetre (potassium nitrate) household ammonia (3-6 mol dm-3 ammonia solution) Teaching Notes Compounds essential for healthy plant growth are made from one or more atoms of certain elements. The three elements whose compounds are need in the largest quantities are: Nitrogen, N; Phosphorous, P; Potassium, K. The next three needed, but in small quantities are Sulfur, S; Magnesium, Mg; Calcium, Ca. Plants also need much smaller quantities of compounds of several other elements. This activity will help students make connections between everyday materials and chemical compounds found in the laboratory. It is an opportunity to discuss, for example, compounds, mixture, purity, concentration, accuracy and precision. Activity 1: ‘Homemade plant food’ There are various types of seed meal. In the UK flaxseed meal can be bought from suppliers such as ‘The Cherry Mill’. However, make sure you buy ‘flaxseed meal’ and not ‘flaxseed’ (the latter is found, for example, in health food shops). You could discuss what an ‘energy store’ is for plants. All substances store energy by virtue of the movement of their particles and the forces of attraction between them (kinetic energy and potential energy). However, to be ‘food’ there must be a way that the stored energy can be released. Plants can releases stored energy from glucose (by respiration), but not from plant nutrients. Answers 1. It is not an energy store that can be used by plants. 2. Flax seeds are crushed, the oil is extracted and what remains is flaxseed meal. 3. (a) calcium carbonate, (b) calcium sulfate, (c) a mixture of calcium carbonate and magnesium carbonate 4. Sulfur. 5. To ensure that whatever quantity is taken it contains the same proportions of ingredients. If it didn’t some plants would get too little of an ingredient and others too much of the ingredient. Science & Plants for Schools: www.saps.org.uk Homemade Fertilisers: p. 2 6. Measuring mass requires a balance and takes time. Measuring volume requires simple apparatus, e.g. such as a spoon or a cup, and is quicker. Activity 2: ‘Epsom salts plant fertiliser’ This activity is an opportunity to differentiate between a mixture and a pure chemical substance (element or compound). There is also an opportunity to discuss measurement units and the advantages and disadvantages of using teaspoons or tablespoons for measuring quantities. Answers 1. (a) Baking powder is a mixture of sodium hydrogen carbonate and flour, (b) Epsom salts is a chemical compound called magnesium sulfate, (c) Saltpetre is an old name for the compound potassium nitrate. 2. 5-10% w/v (5-10 g of ammonia in 100 g of water) 3. (a) About 5.7 g, (b) about 4.9 cm 3, (c) 3785 cm3. Activity 3: Testing ‘Epsom salts plant fertiliser’ This is predominately a planning exercise, but if time allows you could ask students to carry out their planned investigation, having first checked them and carried out a risk assessment. If students do try it, the quantities can be scaled down. Science & Plants for Schools: www.saps.org.uk Homemade Fertilisers: p. 3